Sat, Feb 21, 2026
| فارسی
Volume 31, Issue 1 (Continuously Updated 2025)
IJPCP 2025, 31(1): 0-0 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Jalili Khiavi P, Rasouli A, Ghafari F, Shabani M, Saed O. Effects of tDCS on Positive, Negative, and Depressive Symptoms in Schizophrenic Patients With Predominant Negative Symptoms: A Sham-controlled Clinical Trial. IJPCP 2025; 31 (1)
URL: http://ijpcp.iums.ac.ir/article-1-4474-en.html
URL: http://ijpcp.iums.ac.ir/article-1-4474-en.html
1- Department of Psychiatry, School of Medicine, Zanjan University of Medical Sciences, Zanjan, Iran.
2- Department of Clinical Psychology, Student Research Committee, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
3- Department of Clinical Psychology, School of Medicine, Zanjan University of Medical Sciences, Zanjan, Iran.
4- Department of Clinical Psychology, School of Medicine, Zanjan University of Medical Sciences, Zanjan, Iran. ,o.saed@zums.ac.ir
2- Department of Clinical Psychology, Student Research Committee, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
3- Department of Clinical Psychology, School of Medicine, Zanjan University of Medical Sciences, Zanjan, Iran.
4- Department of Clinical Psychology, School of Medicine, Zanjan University of Medical Sciences, Zanjan, Iran. ,
Keywords: Schizophrenia, Transcranial direct current stimulation (tDCS), Dorsolateral prefrontal cortex (DLPFC), Depression
Full-Text [PDF 6210 kb]
(118 Downloads)
| Abstract (HTML) (346 Views)
Full-Text: (90 Views)
Introduction
Schizophrenia affects approximately 0.5-1% of the global population, with a prevalence increased from 14.2 million cases in 1990 to 23.6 million in 2019, highlighting its growing public health concern. While positive symptoms (hallucinations, delusions) generally respond to antipsychotic medications, negative symptoms (blunted affect, anhedonia, alogia, asociality, avolition) remain largely treatment-resistant despite their profound impact on functional outcomes and quality of life. Evidence indicates that approximately 30-40% of patients experience persistent negative symptoms despite appropriate pharmacological treatment.
Neurobiological research has consistently demonstrated functional abnormalities in the dorsolateral prefrontal cortex (DLPFC) in schizophrenia patients. The phenomenon of hypofrontality (reduced activity in the frontal brain regions) correlates significantly with negative symptom severity, with greater activity reduction associated with more severe symptoms. Given the key role of DLPFC in negative symptom pathophysiology, non-invasive brain stimulation techniques have emerged as potential therapeutic approaches. One of these methods is transcranial direct current stimulation (tDCS), which uses weak electrical current to modulate cortical excitability and may address the interhemispheric imbalance and hypofrontality observed in schizophrenia.
While international evidence shows tDCS efficacy for negative symptoms, research in Iranian populations remains limited. This study aimed to investigate the effects of tDCS on positive, negative, and depressive symptoms in schizophrenia patients with predominant negative symptoms. We hypothesized that anodal stimulation of the left DLPFC and cathodal stimulation of the right orbitofrontal cortex significantly improve negative symptoms compared to sham tDCS, with effects persisting for one month.
Method
This double-blind, sham-controlled randomized clinical trial was conducted on 40 patients aged 18-65 years diagnosed with schizophrenia having predominant negative symptoms. Sample size was calculated using G*Power software, version 3.1.9.7 and based on previous studies, considering an effect size of 0.9, test power of 0.80, and significance level of 0.05. Accounting for a potential 10% dropout rate, the final sample size was determined to be 40 (20 per group). Participants were recruited via convenience sampling from among inpatients and outpatients of Shahid Beheshti Hospital in Zanjan, Iran.
Inclusion criteria were age 18-65 years, diagnosis of schizophrenia with predominant negative symptoms according to the DSM-5 criteria, and stable antipsychotic medication uptake for at least four months. Exclusion criteria were substance dependence, comorbid psychiatric disorders, neurological diseases, pregnancy, severe physical illnesses, having implants, and scalp skin diseases.
The patients were randomly assigned to either active tDCS (n=20) or sham tDCS (n=20) using the block randomization method. The intervention consisted of 15 daily sessions of tDCS. In the active group, the anode was placed over the left DLPFC (F3) and the cathode over the right orbitofrontal region (Fp2) and a 2mA current was applied for 20 minutes. In the sham group, electrode placement was identical, but the device automatically turned off after 30 seconds. Assessments were done using the positive and negative syndrome scale (PANSS), scale for the assessment of negative symptoms (SANS), and Calgary depression scale (CDSS) at baseline, post-intervention, and one-month follow-up by a blinded clinical psychologist.
Results
There was an equal gender distribution in both groups (50% male, 50% female). The mean age in the active tDCS group was 40.40±8.00 years and in the sham group 44.70±8.42 years. Statistical tests showed no significant differences between groups in demographic variables (P>0.05), indicating homogeneous groups at baseline. Independent t-tests confirmed no significant differences between groups in pretest scores for positive symptoms (t=0.05, P=0.962), negative symptoms (t=-0.41, P=0.683), and depression (t=1.73, P=0.92), demonstrating balanced symptom severity at baseline.
Repeated measures ANOVA showed significant changes in dependent variables across the three time points in both groups (P<0.05). However, the effect size was substantially larger in the active tDCS group compared to the sham group. In the active tDCS group, 78.5% of the variance in positive symptoms (F=69.40, P<0.001, η²=0.785), 68.1% of the variance in negative symptoms (F=40.59, P<0.001, η²=0.681), and 33.6% of the variance in depression (F=9.62, P<0.001, η²=0.336) was explained by the intervention (Table 1).
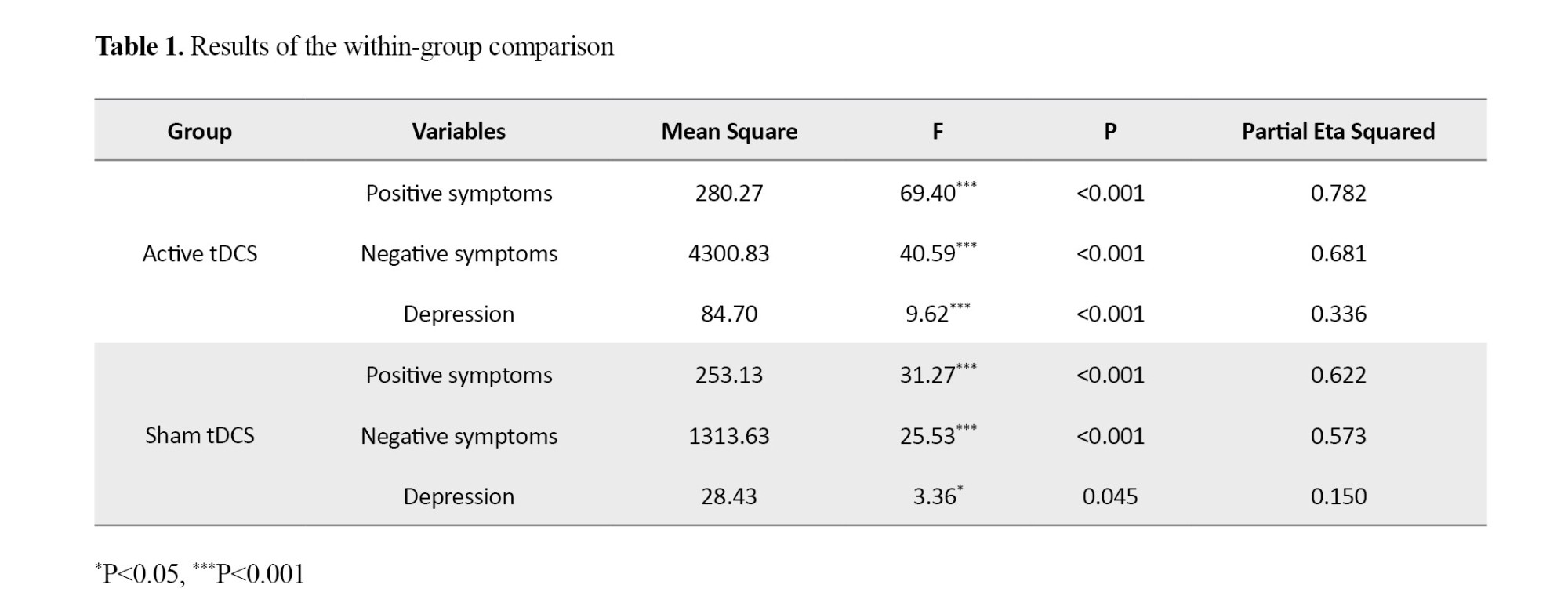
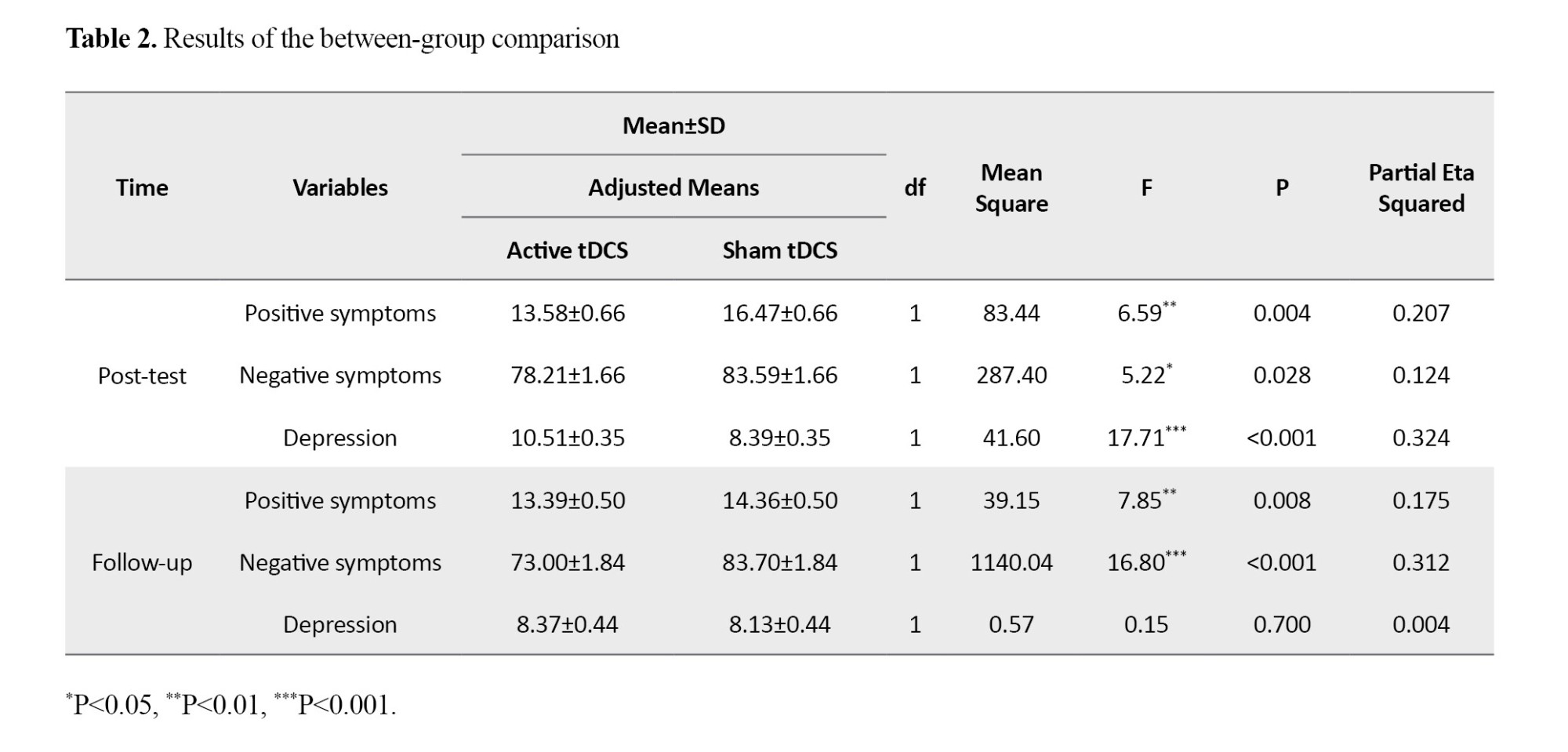
ANCOVA results showed that after controlling for baseline scores, the active tDCS group demonstrated significant reductions in positive symptoms (F=6.59, P=0.004, η²=0.207) and negative symptoms (F=5.22, P=0.028, η²=0.124) in the post-test phase compared to the sham group (Table 2). These therapeutic effects persisted for one month only on positive and negative symptoms.
Conclusions
This study demonstrated that tDCS significantly improved negative and positive symptoms and depression in patients with schizophrenia, with therapeutic effects persisting for one month only on negative and positive symptoms. Despite promising findings, limitations of this study included a relatively small sample size, short follow-up period, and lack of assessment of cognitive function and quality of life. In conclusion, tDCS is an effective, safe, and non-invasive method for improving negative and positive symptoms in schizophrenia, suggesting its potential as an adjunctive treatment along with antipsychotic medications.
Ethical Considerations
Compliance with ethical guidelines
The study received approval from the Ethics Committee of Zanjan University of Medical Sciences (Code: IR.ZUMS.REC.1398.102) and was registered by the Iranian Registry of Clinical Trials (ID: IRCT20200203046366N1).
Funding
This study was funded by Zanjan University of Medical Sciences, Zanjan, Iran.
Authors contributions
Conceptualization, Paria Jalili Khiavi, Mina Shabani, Omid Saed; Methodology, Omid Saed; Formal Analysis, Amirhossein Rasouli; Investigation, Fatemeh Ghaffari; Writing – Original Draft Preparation, Paria Jalili Khiavi, Amirhossein Rasouli; Writing – Review & Editing, all author; Supervision, Mina Shabani, Omid Saed; Validation, All Authors.
Conflicts of interest
The authors declared no conflict of interest.
Acknowledgments
"We would like to thank all participants for their participation in this research.
Schizophrenia affects approximately 0.5-1% of the global population, with a prevalence increased from 14.2 million cases in 1990 to 23.6 million in 2019, highlighting its growing public health concern. While positive symptoms (hallucinations, delusions) generally respond to antipsychotic medications, negative symptoms (blunted affect, anhedonia, alogia, asociality, avolition) remain largely treatment-resistant despite their profound impact on functional outcomes and quality of life. Evidence indicates that approximately 30-40% of patients experience persistent negative symptoms despite appropriate pharmacological treatment.
Neurobiological research has consistently demonstrated functional abnormalities in the dorsolateral prefrontal cortex (DLPFC) in schizophrenia patients. The phenomenon of hypofrontality (reduced activity in the frontal brain regions) correlates significantly with negative symptom severity, with greater activity reduction associated with more severe symptoms. Given the key role of DLPFC in negative symptom pathophysiology, non-invasive brain stimulation techniques have emerged as potential therapeutic approaches. One of these methods is transcranial direct current stimulation (tDCS), which uses weak electrical current to modulate cortical excitability and may address the interhemispheric imbalance and hypofrontality observed in schizophrenia.
While international evidence shows tDCS efficacy for negative symptoms, research in Iranian populations remains limited. This study aimed to investigate the effects of tDCS on positive, negative, and depressive symptoms in schizophrenia patients with predominant negative symptoms. We hypothesized that anodal stimulation of the left DLPFC and cathodal stimulation of the right orbitofrontal cortex significantly improve negative symptoms compared to sham tDCS, with effects persisting for one month.
Method
This double-blind, sham-controlled randomized clinical trial was conducted on 40 patients aged 18-65 years diagnosed with schizophrenia having predominant negative symptoms. Sample size was calculated using G*Power software, version 3.1.9.7 and based on previous studies, considering an effect size of 0.9, test power of 0.80, and significance level of 0.05. Accounting for a potential 10% dropout rate, the final sample size was determined to be 40 (20 per group). Participants were recruited via convenience sampling from among inpatients and outpatients of Shahid Beheshti Hospital in Zanjan, Iran.
Inclusion criteria were age 18-65 years, diagnosis of schizophrenia with predominant negative symptoms according to the DSM-5 criteria, and stable antipsychotic medication uptake for at least four months. Exclusion criteria were substance dependence, comorbid psychiatric disorders, neurological diseases, pregnancy, severe physical illnesses, having implants, and scalp skin diseases.
The patients were randomly assigned to either active tDCS (n=20) or sham tDCS (n=20) using the block randomization method. The intervention consisted of 15 daily sessions of tDCS. In the active group, the anode was placed over the left DLPFC (F3) and the cathode over the right orbitofrontal region (Fp2) and a 2mA current was applied for 20 minutes. In the sham group, electrode placement was identical, but the device automatically turned off after 30 seconds. Assessments were done using the positive and negative syndrome scale (PANSS), scale for the assessment of negative symptoms (SANS), and Calgary depression scale (CDSS) at baseline, post-intervention, and one-month follow-up by a blinded clinical psychologist.
Results
There was an equal gender distribution in both groups (50% male, 50% female). The mean age in the active tDCS group was 40.40±8.00 years and in the sham group 44.70±8.42 years. Statistical tests showed no significant differences between groups in demographic variables (P>0.05), indicating homogeneous groups at baseline. Independent t-tests confirmed no significant differences between groups in pretest scores for positive symptoms (t=0.05, P=0.962), negative symptoms (t=-0.41, P=0.683), and depression (t=1.73, P=0.92), demonstrating balanced symptom severity at baseline.
Repeated measures ANOVA showed significant changes in dependent variables across the three time points in both groups (P<0.05). However, the effect size was substantially larger in the active tDCS group compared to the sham group. In the active tDCS group, 78.5% of the variance in positive symptoms (F=69.40, P<0.001, η²=0.785), 68.1% of the variance in negative symptoms (F=40.59, P<0.001, η²=0.681), and 33.6% of the variance in depression (F=9.62, P<0.001, η²=0.336) was explained by the intervention (Table 1).

ANCOVA results showed that after controlling for baseline scores, the active tDCS group demonstrated significant reductions in positive symptoms (F=6.59, P=0.004, η²=0.207) and negative symptoms (F=5.22, P=0.028, η²=0.124) in the post-test phase compared to the sham group (Table 2). These therapeutic effects persisted for one month only on positive and negative symptoms.

Conclusions
This study demonstrated that tDCS significantly improved negative and positive symptoms and depression in patients with schizophrenia, with therapeutic effects persisting for one month only on negative and positive symptoms. Despite promising findings, limitations of this study included a relatively small sample size, short follow-up period, and lack of assessment of cognitive function and quality of life. In conclusion, tDCS is an effective, safe, and non-invasive method for improving negative and positive symptoms in schizophrenia, suggesting its potential as an adjunctive treatment along with antipsychotic medications.
Ethical Considerations
Compliance with ethical guidelines
The study received approval from the Ethics Committee of Zanjan University of Medical Sciences (Code: IR.ZUMS.REC.1398.102) and was registered by the Iranian Registry of Clinical Trials (ID: IRCT20200203046366N1).
Funding
This study was funded by Zanjan University of Medical Sciences, Zanjan, Iran.
Authors contributions
Conceptualization, Paria Jalili Khiavi, Mina Shabani, Omid Saed; Methodology, Omid Saed; Formal Analysis, Amirhossein Rasouli; Investigation, Fatemeh Ghaffari; Writing – Original Draft Preparation, Paria Jalili Khiavi, Amirhossein Rasouli; Writing – Review & Editing, all author; Supervision, Mina Shabani, Omid Saed; Validation, All Authors.
Conflicts of interest
The authors declared no conflict of interest.
Acknowledgments
"We would like to thank all participants for their participation in this research.
References
- Cheng PWC, Louie LLC, Wong YL, Wong SMC, Leung WY, Nitsche MA, et al. The effects of transcranial direct current stimulation (tDCS) on clinical symptoms in schizophrenia: A systematic review and meta-analysis. Asian Journal of Psychiatry. 2020; 53:102392. [DOI:10.1016/j.ajp.2020.102392] [PMID]
- Collaborators GMD. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990-2019: A systematic analysis for the global burden of disease study 2019. The Lancet Psychiatry. 2022; 9(2):137-50. [DOI:10.1016/S2215-0366(21)00395-3] [PMID]
- Pontillo M, Costanzo F, Menghini D, Averna R, Santonastaso O, Tata MC, et al. Use of transcranial direct stimulation in the treatment of negative symptoms of schizophrenia. Clinical EEG and Neuroscience. 2018; 49(1):18-26. [DOI:10.1177/1550059417746531] [PMID]
- McCutcheon RA, Reis Marques T, Howes OD. Schizophrenia-An overview. JAMA Psychiatry. 2020; 77(2):201-10. [DOI:10.1001/jamapsychiatry.2019.3360] [PMID]
- Kirkpatrick B, Fenton WS, Carpenter WT, Marder SR. The NIMH-MATRICS consensus statement on negative symptoms. Schizophrenia Bulletin. 2006; 32(2):214-9. [DOI:10.1093/schbul/sbj053] [PMID]
- Aleman A, Enriquez-Geppert S, Knegtering H, Dlabac-de Lange JJ. Moderate effects of noninvasive brain stimulation of the frontal cortex for improving negative symptoms in schizophrenia: Meta-analysis of controlled trials. Neuroscience & Biobehavioral Reviews. 2018; 89:111-8. [DOI:10.1016/j.neubiorev.2018.02.009] [PMID]
- Sadock BJ, Sadock VA. Kaplan & Sadock's comprehensive textbook of psychiatry. Philadelphia: Lippincott Williams & Wilkins Publishers; 2000.
- Osoegawa C, Gomes JS, Grigolon RB, Brietzke E, Gadelha A, Lacerda AL, et al. Non-invasive brain stimulation for negative symptoms in schizophrenia: An updated systematic review and meta-analysis. Schizophrenia Research. 2018; 197:34-44. [DOI:10.1016/j.schres.2018.01.010] [PMID]
- Palm U, Keeser D, Hasan A, Kupka MJ, Blautzik J, Sarubin N, et al. Prefrontal transcranial direct current stimulation for treatment of schizophrenia with predominant negative symptoms: A double-blind, sham-controlled proof-of-concept study. Schizophrenia Bulletin. 2016; 42(5):1253-61. [DOI:10.1093/schbul/sbw041] [PMID]
- Aleman A, Lincoln TM, Bruggeman R, Melle I, Arends J, Arango C, et al. Treatment of negative symptoms: Where do we stand, and where do we go? Schizophrenia Research. 2017; 186:55-62. [DOI:10.1016/j.schres.2016.05.015] [PMID]
- Krause M, Zhu Y, Huhn M, Schneider-Thoma J, Bighelli I, Nikolakopoulou A, et al. Antipsychotic drugs for patients with schizophrenia and predominant or prominent negative symptoms: A systematic review and meta-analysis. European Archives of Psychiatry and Clinical Neuroscience. 2018; 268(7):625-39. [DOI:10.1007/s00406-018-0869-3] [PMID]
- Farnia S, Mahtiyan E, Zarghami M, Eslami Parkoohi P, Emadian A, Hendouei N. Evaluation of the efficacy and safety of adding pregabalin to antipsychotic treatment in patients with chronic schizophrenia: A double-blind placebo-controlled clinical trial. Iranian Journal of Psychiatry and Clinical Psychology. 2022; 27(4):428-39. [DOI:10.32598/ijpcp.27.3.2167.3]
- Gomes JS, Shiozawa P, Dias ÁM, Ducos DV, Akiba H, Trevizol AP, et al. Left dorsolateral prefrontal cortex anodal tDCS effects on negative symptoms in schizophrenia. Brain Stimulation: Basic, Translational, and Clinical Research in Neuromodulation. 2015; 8(5):989-91. [DOI:10.1016/j.brs.2015.07.033] [PMID]
- Correll CU, Schooler NR. Negative symptoms in schizophrenia: A review and clinical guide for recognition, assessment, and treatment. Neuropsychiatric Disease and Treatment. 2020; 16:519. [DOI:10.2147/NDT.S225643] [PMID]
- Zhuo K, Tang Y, Song Z, Wang Y, Wang J, Qian Z, et al. Repetitive transcranial magnetic stimulation as an adjunctive treatment for negative symptoms and cognitive impairment in patients with schizophrenia: A randomized, double-blind, sham-controlled trial. Neuropsychiatric Disease and Treatment. 2019; 15:1141. [DOI:10.2147/NDT.S196086] [PMID]
- McTeague LM, Huemer J, Carreon DM, Jiang Y, Eickhoff SB, Etkin A. Identification of common neural circuit disruptions in cognitive control across psychiatric disorders. American Journal of Psychiatry. 2017; 174(7):676-85. [DOI:10.1176/appi.ajp.2017.16040400] [PMID]
- Núñez C, Paipa N, Senior C, Coromina M, Siddi S, Ochoa S, et al. Global brain asymmetry is increased in schizophrenia and related to avolition. Acta Psychiatrica Scandinavica. 2017; 135(5):448-59. [DOI:10.1111/acps.12723] [PMID]
- Wolkin A, Sanfilipo M, Wolf AP, Angrist B, Brodie JD, Rotrosen J. Negative symptoms and hypofrontality in chronic schizophrenia. Archives of General Psychiatry. 1992; 49(12):959-65. [DOI:10.1001/archpsyc.1992.01820120047007] [PMID]
- Huang ML, Khoh TT, Lu SJ, Pan F, Chen JK, Hu JB, et al. Relationships between dorsolateral prefrontal cortex metabolic change and cognitive impairment in first-episode neuroleptic-naive schizophrenia patients. Medicine. 2017; 96(25):e7228. [DOI:10.1097/MD.0000000000007228] [PMID]
- Chechko N, Cieslik EC, Müller VI, Nickl-Jockschat T, Derntl B, Kogler L, et al. Differential resting-state connectivity patterns of the right anterior and posterior dorsolateral prefrontal cortices (DLPFC) in Schizophrenia. Frontiers in Psychiatry. 2018; 9:211. [DOI:10.3389/fpsyt.2018.00211] [PMID]
- Fröhlich F, Jarskog LF. 1136non-invasive brain stimulation in schizophrenia. In: Wassermann EM, Peterchev AV, Ziemann U, Lisanby SH, Siebner HR, Walsh V, editors. The Oxford handbook of transcranial stimulation: Second edition. Oxford: Oxford University Press; 2024. [DOI:10.1093/oxfordhb/9780198832256.013.43]
- Xiu MH, Guan HY, Zhao JM, Wang KQ, Pan YF, Su XR, et al. Cognitive enhancing effect of high-frequency neuronavigated rTMS in chronic schizophrenia patients with predominant negative symptoms: A double-blind controlled 32-week follow-up study. Schizophrenia Bulletin. 2020; 46(5):1219-30. [DOI:10.1093/schbul/sbaa035] [PMID]
- Kim J, Iwata Y, Plitman E, Caravaggio F, Chung JK, Shah P, et al. A meta-analysis of transcranial direct current stimulation for schizophrenia:”Is more better? Journal of Psychiatric Research. 2019; 110:117-26. [DOI:10.1016/j.jpsychires.2018.12.009] [PMID]
- Brunelin J, Mondino M, Gassab L, Haesebaert F, Gaha L, Suaud-Chagny MF, et al. Examining transcranial direct-current stimulation (tDCS) as a treatment for hallucinations in schizophrenia. American Journal of Psychiatry. 2012; 169(7):719-24. [DOI:10.1176/appi.ajp.2012.11071091]
- Mondino M, Brunelin J, Palm U, R. Brunoni A, Poulet E, Fecteau S. Transcranial direct current stimulation for the treatment of refractory symptoms of schizophrenia. Current evidence and future directions. Current Pharmaceutical Design. 2015; 21(23):3373-83. [DOI:10.2174/1381612821666150619093648] [PMID]
- Omranifard V, Pourabadei P, Asgari K. The effectiveness of Transcranial Direct- Current Stimulation (tDCS) combined with medication on negative symptoms of schizophrenic patients. Shenakht Journal of Psychology and Psychiatry. 2019; 6(2):38-61. [DOI:10.29252/shenakht.6.2.38]
- Pondé PH, de Sena EP, Camprodon JA, de Araújo AN, Neto MF, DiBiasi M, et al. Use of transcranial direct current stimulation for the treatment of auditory hallucinations of schizophrenia - a systematic review. Neuropsychiatric Disease and Treatment. 2017;13:347-55. [DOI:10.2147/NDT.S122016] [PMID]
- Chang CC, Kao YC, Chao CY, Tzeng NS, Chang HA. Examining bi-anodal transcranial direct current stimulation (tDCS) over bilateral dorsolateral prefrontal cortex coupled with bilateral extracephalic references as a treatment for negative symptoms in non-acute schizophrenia patients: A randomized, double-blind, sham-controlled trial. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2020; 96:109715. [DOI:10.1016/j.pnpbp.2019.109715] [PMID]
- Ghamari Givi H, Moulavi P, Heshmati R. [Exploration of the factor structure of positive and negative syndrome scale in schizophernia spectrum disorder (Persian)]. Journal of Clinical Psychology. 2010; 2(2):1-10. [DOI:10.22075/jcp.2017.2018]
- Andreasen NC. The scale for the assessment of negative symptoms (SANS): Conceptual and theoretical foundations. The British Journal of Psychiatry. 1989; 155(S7):49-52. [DOI:10.1192/S0007125000291496]
- Javanmard G. [Comparison of social judgment ability in schizophrenic patients with negative and positive symptoms and a healthy group (Persian)]. Journal of Modern Psychological Researches. 2019; 14(54):57-74. [Link]
- Addington D, Addington J, Schissel B. A depression rating scale for schizophrenics. Schizophrenia Research. 1990; 3(4):247-51. [DOI:10.1016/0920-9964(90)90005-R] [PMID]
- Valiengo L, Gordon PC, de Carvalho JB, Rios RM, Koebe S, Serpa MH, et al. Schizophrenia treatment with electric transcranial stimulation (STARTS): Design, rationale and objectives of a randomized, double-blinded, sham-controlled trial. Trends in Psychiatry and Psychotherapy. 2019; 41(2):104-11. [DOI:10.1590/2237-6089-2018-0047] [PMID]
- Lindenmayer J, Kulsa MKC, Sultana T, Kaur A, Yang R, Ljuri I, et al. Transcranial direct-current stimulation in ultra-treatment-resistant schizophrenia. Brain Stimulation. 2019; 12(1):54-61. [DOI:10.1016/j.brs.2018.10.002] [PMID]
- He W, Fong PY, Leung TWH, Huang YZ. Protocols of non-invasive brain stimulation for neuroplasticity induction. Neuroscience Letters. 2020; 719:133437. [DOI:10.1016/j.neulet.2018.02.045] [PMID]
- Hill K, Mann L, Laws K, Stephenson C, Nimmo‐Smith I, McKenna P. Hypofrontality in schizophrenia: A meta‐analysis of functional imaging studies. Acta Psychiatrica Scandinavica. 2004; 110(4):243-56. [DOI:10.1111/j.1600-0447.2004.00376.x] [PMID]
- Shaffer JJ, Peterson MJ, McMahon MA, Bizzell J, Calhoun V, van Erp TG, et al. Neural correlates of schizophrenia negative symptoms: Distinct subtypes impact dissociable brain circuits. Molecular Neuropsychiatry. 2015; 1(4):191-200. [DOI:10.1159/000440979] [PMID]
- Lee TY, Lee J, Kim M, Kwon JS. The effect of transcranial direct current stimulation on auditory hallucination in patients with schizophrenia. Schizophrenia Research. 2018; 192:489-490. [DOI:10.1016/j.schres.2017.06.012] [PMID]
Type of Study: Original Research |
Subject:
Psychiatry and Psychology
Received: 2025/03/18 | Accepted: 2025/10/2 | Published: 2025/10/28
Received: 2025/03/18 | Accepted: 2025/10/2 | Published: 2025/10/28
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






