Wed, Dec 24, 2025
| فارسی
Volume 30, Issue 1 (Continuously Updated 2024)
IJPCP 2024, 30(1): 0-0 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Rabiee S, Heysieattalab S, Vahabi Z, Sabaghypour S. A Comparative Study on the Working Memory of the Children of Parents With and Without Alzheimer's Disease. IJPCP 2024; 30 (1) : 4871.1
URL: http://ijpcp.iums.ac.ir/article-1-4091-en.html
URL: http://ijpcp.iums.ac.ir/article-1-4091-en.html
1- Department of Cognitive Neuroscience, Faculty of Education and Psychology, University of Tabriz, Tabriz, Iran.
2- Department of Cognitive Neuroscience, Faculty of Education and Psychology, University of Tabriz, Tabriz, Iran. ,heysieattalab@gmail.com
3- Department of Psychiatry, Faculty of Medicine, Roozbeh Hospital and Geriatric Department, Tehran University of Medical Sciences, Tehran, Iran.
4- Department of Cognitive Neuroscience, Faculty of Education and Psychology, University of Tabriz, Tabriz, Iran
2- Department of Cognitive Neuroscience, Faculty of Education and Psychology, University of Tabriz, Tabriz, Iran. ,
3- Department of Psychiatry, Faculty of Medicine, Roozbeh Hospital and Geriatric Department, Tehran University of Medical Sciences, Tehran, Iran.
4- Department of Cognitive Neuroscience, Faculty of Education and Psychology, University of Tabriz, Tabriz, Iran
Keywords: Cognitive function, Working memory, Alzheimer’s disease extended abstract, Children of AD, SWM, PAL, PRM
Full-Text [PDF 6113 kb]
(740 Downloads)
| Abstract (HTML) (2222 Views)
Full-Text: (743 Views)
Introduction
Alzheimer's disease (AD) is one of common neurodegenerative disorders, characterized by a slowly progressive loss of memory and cognitive impairments. Some brain regions, notably the temporal areas, are involved in AD. These affected brain regions are related to cognitive functions such as spatial working memory (SWM), pattern recognition memory (PRM), and paired associates learning (PAL). These cognitive processes play crucial roles in daily functioning. This study aims to assess the performance of these three cognitive functions in the children of parents diagnosed with AD and healthy parents using the Cambridge neuropsychological test automated battery (CANTAB). By comparing the cognitive performance between these groups, we aimed to identify potential early markers that can be indicatives of the AD risk.
Method
In this comparative study, participants were 55 children, 31 children of parents diagnosed with AD and 24 children of healthy parents, recruited from a neurology clinic in Tehran, Iran. Inclusion criteria for the healthy group were having parents with no history of traumatic brain injury, major psychiatric disorders, vascular dementia, brain tumors, neurological disorders (such as Parkinson's disease), or orthopedic disorders that could impede test performance. Additionally, participants underwent neurocognitive tests to ensure cognitive competence for the study assessments. The tests assessed three cognitive domains: SWM, PRM, and PAL utilizing the CANTAB method. These tests were administered under controlled conditions, ensuring standardized testing procedures for all participants. Statistical analyses were performed in SPSS using independent t-test and Mann-Whitney U test to examine the differences in cognitive performance between the two groups.
Results
Table 1 shows the mean scores of SWM, PRM, and PAL in two study groups. Regarding the SWM, there were significant differences between the two groups in the items measuring between errors and total errors.
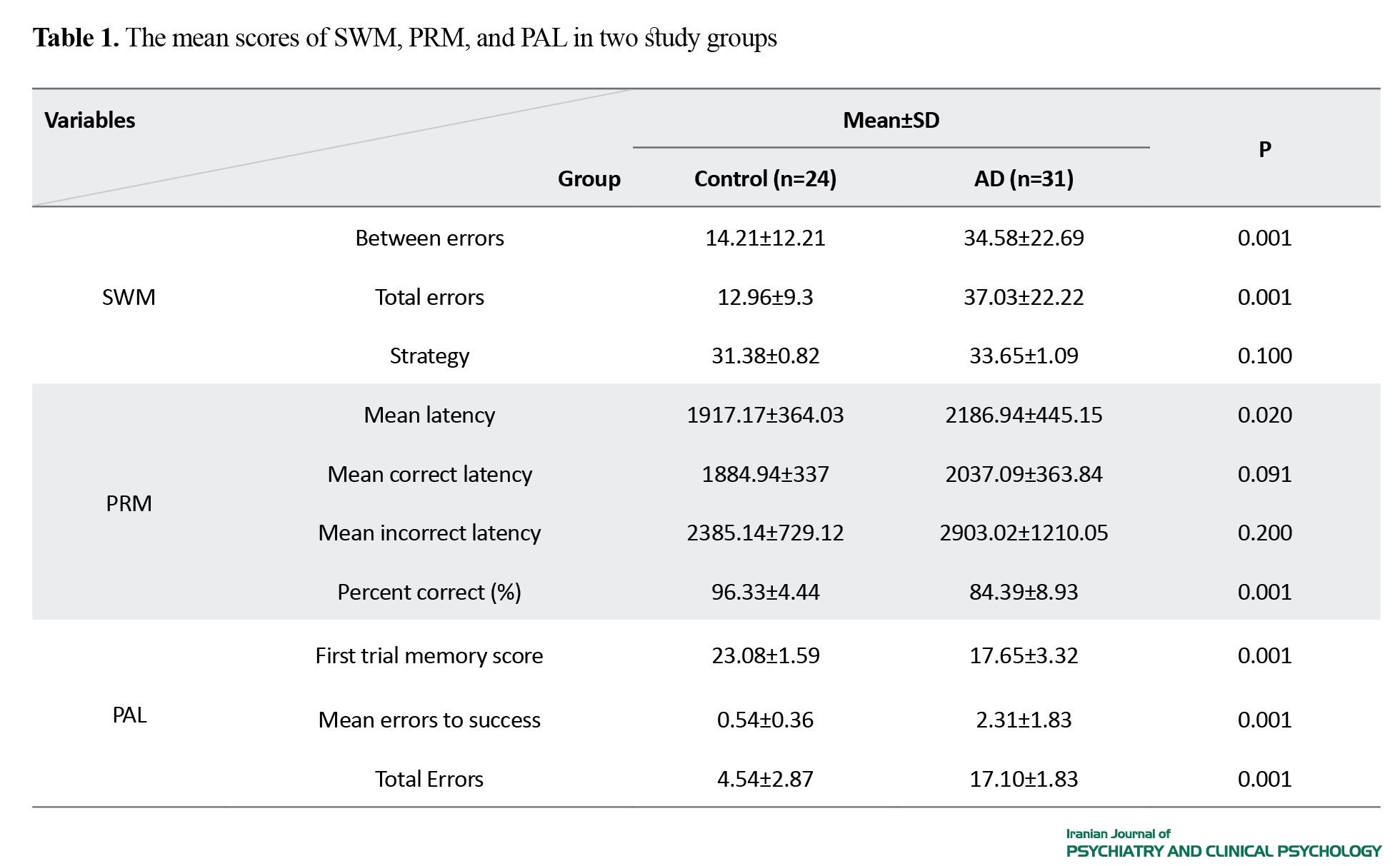
These differences indicated a distinctive SWM domain among the children of AD patients. The PRM did not show significant differences between the two groups in mean correct latency and mean incorrect latency (P>0.05), but the difference was significant in mean latency and percent correct (P<0.05). This suggests a comparable PRM between the children of AD and healthy parents. The PAL performance demonstrated significant differences in all three parameters (first trial memory score, mean errors to success, and total errors) assessed between the two groups (P<0.01). These findings indicate potential cognitive markers associated with AD susceptibility among the children of affected parents.
Conclusion
The observed differences in SWM and PAL between the children of AD parents and healthy parents can indicate the factors that may be associated with the AD risk. However, the absence of significant differences in PRM between the two groups in mean correct and incorrect latencies. Although this cognitive domain did not exhibit significant difference, it remains crucial to conduct more studies to assess its association with AD. In this regard, further longitudinal studies involving a different cognitive functions are recommended to expand these findings for more robust predictive models of AD onset.
Ethical Considerations
Compliance with ethical guidelines
Participation in this study was voluntary, and informed consent was obtained from all participants after explaining the study objectives and ensuring their confidentiality and the right to leave the study at any time. This study has ethical approval from the ethics committee of the University of Tabriz (Code: IR.TABRIZU.REC.1401.057).
Funding
This article was extracted from the PhD thesis of Sara Rabiei in neuroscience, brain and cognition, at the University of Tabriz. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors contributions
The authors contributed equally to preparing this paper.
Conflicts of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank all participants for their cooperation in this study.
References
Alzheimer's disease (AD) is one of common neurodegenerative disorders, characterized by a slowly progressive loss of memory and cognitive impairments. Some brain regions, notably the temporal areas, are involved in AD. These affected brain regions are related to cognitive functions such as spatial working memory (SWM), pattern recognition memory (PRM), and paired associates learning (PAL). These cognitive processes play crucial roles in daily functioning. This study aims to assess the performance of these three cognitive functions in the children of parents diagnosed with AD and healthy parents using the Cambridge neuropsychological test automated battery (CANTAB). By comparing the cognitive performance between these groups, we aimed to identify potential early markers that can be indicatives of the AD risk.
Method
In this comparative study, participants were 55 children, 31 children of parents diagnosed with AD and 24 children of healthy parents, recruited from a neurology clinic in Tehran, Iran. Inclusion criteria for the healthy group were having parents with no history of traumatic brain injury, major psychiatric disorders, vascular dementia, brain tumors, neurological disorders (such as Parkinson's disease), or orthopedic disorders that could impede test performance. Additionally, participants underwent neurocognitive tests to ensure cognitive competence for the study assessments. The tests assessed three cognitive domains: SWM, PRM, and PAL utilizing the CANTAB method. These tests were administered under controlled conditions, ensuring standardized testing procedures for all participants. Statistical analyses were performed in SPSS using independent t-test and Mann-Whitney U test to examine the differences in cognitive performance between the two groups.
Results
Table 1 shows the mean scores of SWM, PRM, and PAL in two study groups. Regarding the SWM, there were significant differences between the two groups in the items measuring between errors and total errors.

These differences indicated a distinctive SWM domain among the children of AD patients. The PRM did not show significant differences between the two groups in mean correct latency and mean incorrect latency (P>0.05), but the difference was significant in mean latency and percent correct (P<0.05). This suggests a comparable PRM between the children of AD and healthy parents. The PAL performance demonstrated significant differences in all three parameters (first trial memory score, mean errors to success, and total errors) assessed between the two groups (P<0.01). These findings indicate potential cognitive markers associated with AD susceptibility among the children of affected parents.
Conclusion
The observed differences in SWM and PAL between the children of AD parents and healthy parents can indicate the factors that may be associated with the AD risk. However, the absence of significant differences in PRM between the two groups in mean correct and incorrect latencies. Although this cognitive domain did not exhibit significant difference, it remains crucial to conduct more studies to assess its association with AD. In this regard, further longitudinal studies involving a different cognitive functions are recommended to expand these findings for more robust predictive models of AD onset.
Ethical Considerations
Compliance with ethical guidelines
Participation in this study was voluntary, and informed consent was obtained from all participants after explaining the study objectives and ensuring their confidentiality and the right to leave the study at any time. This study has ethical approval from the ethics committee of the University of Tabriz (Code: IR.TABRIZU.REC.1401.057).
Funding
This article was extracted from the PhD thesis of Sara Rabiei in neuroscience, brain and cognition, at the University of Tabriz. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors contributions
The authors contributed equally to preparing this paper.
Conflicts of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank all participants for their cooperation in this study.
References
- Porsteinsson AP, Isaacson RS, Knox S, Sabbagh MN, Rubino I. Diagnosis of early Alzheimer’s disease: Clinical practice in 2021. The Journal of Prevention of Alzheimer’s Disease. 2021; 8:8(3):371-86. [DOI:10.14283/jpad.2021.23] [PMID]
- Mansoori S, Mozaffar F, Noroozian M, Faizi M, Ashayeri H. [Relationship between neuropsychological and physical environmental perception in patients with dementia and alzheimer disease (Persian)]. Iranian Journal of Psychiatry and Clinical Psychology. 2019; 24(4):426-43. [DOI:10.32598/ijpcp.24.4.426]
- Ashiq S, Ashiq K. The association of apolipoprotein-E (APOE) gene polymorphisms with coronary artery disease: A systematic review and meta-analysis. Egyptian Journal of Medical Human Genetics. 2021; 22:16. [DOI:10.1186/s43042-021-00135-2]
- Pourzabih S, Arefnazari M, Tajeri B, Delbari A. The effectiveness of “body-centered meditation” on insomnia and agitation in Alzheimer’s patients. Iranian Journal of Health Psychology. 2021; 4(3):47-54. [Link]
- Jarvik LF, Blazer D. Children of Alzheimer patients: An overview. Journal of Geriatric Psychiatry and Neurology. 2005; 18(4):181-6. [DOI:10.1177/0891988705281859] [PMID]
- Guest FL, Rahmoune H, Guest PC. Early diagnosis and targeted treatment strategy for improved therapeutic outcomes in Alzheimer’s disease. Advances in Experimental Medicine and Biology. 2020; 1260:175-91. [DOI:10.1007/978-3-030-42667-5_8] [PMID]
- Ramos AA, Galiano-Castillo N, Machado L. Cognitive functioning of unaffected first-degree relatives of individuals with late-onset Alzheimer’s Disease: A systematic literature review and meta-analysis. Neuropsychology Review. 2023; 33(4):659-74. [DOI:10.1007/s11065-022-09555-2] [PMID]
- Jarvik L, LaRue A, Blacker D, Gatz M, Kawas C, McArdle JJ, et al. Children of persons with Alzheimer disease: What does the future hold? Alzheimer Disease & Associated Disorders. 2008; 22(1):6-20. [DOI:10.1097/WAD.0b013e31816653ac] [PMID]
- Horvath A, Szucs A, Csukly G, Sakovics A, Stefanics G, Kamondi A. EEG and ERP biomarkers of Alzheimer’s disease: A critical review. Frontiers in Bioscience (Landmark edition). 2018; 23(2):183-220. [DOI:10.2741/4587] [PMID]
- Petersen RC. Clinical practice. Mild cognitive impairment. New England Journal of Medicine. 2011; 364(23):2227-34. [DOI:10.1056/NEJMcp0910237] [PMID]
- Mueller SG, Schuff N, Yaffe K, Madison C, Miller B, Weiner MW. Hippocampal atrophy patterns in mild cognitive impairment and Alzheimer’s disease. Human Brain Mapping. 2010; 31(9):1339-47. [DOI:10.1002/hbm.20934] [PMID]
- Juottonen K, Laakso MP, Insausti R, Lehtovirta M, Pitkänen A, Partanen K, et al. Volumes of the entorhinal and perirhinal cortices in Alzheimer’s disease. Neurobiology of Aging. 1998; 19(1):15-22. [DOI:10.1016/S0197-4580(98)00007-4] [PMID]
- Dhikav V, Anand K. Potential predictors of hippocampal atrophy in Alzheimer’s disease. Drugs & Aging. 2011; 28(1):1-11. [DOI:10.2165/11586390-000000000-00000] [PMID]
- Toepper M, Markowitsch HJ, Gebhardt H, Beblo T, Thomas C, Gallhofer B, et al. Hippocampal involvement in working memory encoding of changing locations: An fMRI study. Brain Research. 2010; 1354:91-9. [DOI:10.1016/j.brainres.2010.07.065] [PMID]
- Wirt RA, Crew LA, Ortiz AA, McNeela AM, Flores E, Kinney JW, et al. Altered theta rhythm and hippocampal-cortical interactions underlie working memory deficits in a hyperglycemia risk factor model of Alzheimer’s disease. Communications Biology. 2021; 4(1):1036. [DOI:10.1038/s42003-021-02558-4] [PMID]
- Spellman T, Rigotti M, Ahmari SE, Fusi S, Gogos JA, Gordon JA. Hippocampal-prefrontal input supports spatial encoding in working memory. Nature. 2015; 522(7556):309-314. [DOI:10.1038/nature14445] [PMID]
- van Ede F, Nobre AC. Turning attention inside out: How working memory serves behavior. Annual Review of Psychology. 2023; 74:137-65. [DOI:10.1146/annurev-psych-021422-041757] [PMID]
- Sheybani F, Aalaei S, Talaei A, Salimi Z, Emran R. [The effect of training working memory and attention control in combination on cravings, impulsivity, and working memory of opiate users under methadone maintenance treatment: A randomized controlled clinical trial (Persian)]. Iranian Journal of Psychiatry and Clinical Psychology. 2024; 29 (4):438-59. [DOI:10.32598/ijpcp.29.4.4643.1]
- Barbeau E, Didic M, Tramoni E, Felician O, Joubert S, Sontheimer A, et al. Evaluation of visual recognition memory in MCI patients. Neurology. 2004; 62(8):1317-22. [DOI:10.1212/01.WNL.0000120548.24298.DB] [PMID]
- Squire LR, Wixted JT, Clark RE. Recognition memory and the medial temporal lobe: A new perspective. Nature Reviews Neuroscience. 2007; 8(11):872-83. [DOI:10.1038/nrn2154] [PMID]
- Sohrabi HR, Ashayeri H, Nasr M. [Retrieving from visual memory in schizophernics right hemisphere brain damaged and normal group (Persian)]. Iranian Journal of Psychiatry and Clinical Psychology. 1998; 4(2):21-31. [Link]
- de Rover M, Pironti VA, McCabe JA, Acosta-Cabronero J, Arana FS, Morein-Zamir S, et al. Hippocampal dysfunction in patients with mild cognitive impairment: A functional neuroimaging study of a visuospatial paired associates learning task. Neuropsychologia. 2011; 49(7):2060-70. [DOI:10.1016/j.neuropsychologia.2011.03.037] [PMID]
- O'Donnell J, Pietrzak RH, Ellis KC, Snyder PJ, Maruff P. Understanding failure of visual paired associate learning in amnestic mild cognitive impairment. Journal of Clinical and Experimental Neuropsychology. 2011; 33(10):1069-78. [DOI:10.1080/13803395.2011.596821] [PMID]
- Rosenberg A, Solomon A, Soininen H, Visser PJ, Blennow K, Hartmann T, et al. Research diagnostic criteria for Alzheimer’s disease: Findings from the LipiDiDiet randomized controlled trial. Alzheimer’s Research & Therapy. 2021; 13(1):64. [DOI:10.1186/s13195-021-00799-3] [PMID]
- Ayodele T, Rogaeva E, Kurup JT, Beecham G, Reitz C. Early-onset Alzheimer’s disease: What is missing in research? Current Neurology and Neuroscience Reports. 2021; 21(2):4. [DOI:10.1007/s11910-020-01090-y] [PMID]
- Rodriguez R, Lopera F, Alvarez A, Fernandez Y, Galan L, Quiroz Y, et al. Spectral analysis of EEG in familial Alzheimer’s disease with E280A presenilin-1 mutation gene. International Journal of Alzheimer’s Disease. 2014; 2014:180741. [DOI:10.1155/2014/180741] [PMID]
- Bucks RS, Nanthakumar S, Starkstein SS, Hillman DR, James A, McArdle N, et al. Discerning depressive symptoms in patients with obstructive sleep apnea: The effect of continuous positive airway pressure therapy on Hamilton Depression Rating Scale symptoms. Sleep. 2018; 41(12):zsy178. [DOI:10.1093/sleep/zsy178]
- Kashani L, Eslatmanesh S, Saedi N, Niroomand N, Ebrahimi M, Hosseinian M, et al. Comparison of saffron versus fluoxetine in treatment of mild to moderate postpartum depression: A double-blind, randomized clinical trial. Pharmacopsychiatry. 2017; 50(2):64-8. [DOI:10.1055/s-0042-115306] [PMID]
- Foroughan M, Jafari Z, Shirin Bayan P, Ghaem Magham Farahani Z, Rahgozar M. [Validation of Mini- Mental State Examination (MMSE) in the elderly population of Tehran (Persian)]. Advances in Cognitive Sciences. 2008; 10(2):29-37. [Link]
- Arevalo-Rodriguez I, Smailagic N, i Figuls MR, Ciapponi A, Sanchez-Perez E, Giannakou A, et al. Mini-Mental State Examination (MMSE) for the detection of Alzheimer’s disease and other dementias in people with mild cognitive impairment (MCI). Cochrane Database of Systematic Reviews. 2015. [DOI:10.1002/14651858.CD010783.pub2]
- Mathuranath PS, Nestor PJ, Berrios GE, Rakowicz W, Hodges JR. A brief cognitive test battery to differentiate Alzheimer’s disease and frontotemporal dementia. Neurology. 2000; 55(11):1613-20. [DOI:10.1212/01.wnl.0000434309.85312.19] [PMID]
- Pouretemad HR, Khatibi A, Ganjavi A, Shams J, Zarei M. Validation of Addenbrooke’s cognitive examination (ACE) in a Persian-speaking population. Dementia and Geriatric Cognitive Disorders. 2009; 28(4):343-7. [DOI:10.1159/000252772] [PMID]
- Lenehan ME, Summers MJ, Saunders NL, Summers JJ, Vickers JC. Does the Cambridge Automated Neuropsychological Test Battery (CANTAB) distinguish between cognitive domains in healthy older adults? Assessment. 2016; 23(2):163-72. [DOI:10.1177/1073191115581474] [PMID]
- Cacciamani F, Salvadori N, Eusebi P, Lisetti V, Luchetti E, Calabresi P, et al. Evidence of practice effect in CANTAB spatial working memory test in a cohort of patients with mild cognitive impairment. Applied Neuropsychology: Adult. 2018; 25(3):237-48. [DOI:10.1080/23279095.2017.1286346] [PMID]
- Soares FC, de Oliveira TC, de Macedo LD, Tomás AM, Picanço-Diniz DL, Bento-Torres J, et al. CANTAB object recognition and language tests to detect aging cognitive decline: An exploratory comparative study. Clinical Interventions in Aging.2014; 10:37-48. [DOI:10.2147/CIA.S68186] [PMID]
- Balbaid NT, Al-Dawalibi A, Khattab AM, Al-Saqr F, AbuSittah A, Alqarni S, et al. The relationship between cognitive impairment and coronary artery disease in middle-aged adults. Cureus. 2020; 12(1):e6724. [DOI:10.7759/cureus.6724] [PMID]
- Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. New England Journal of Medicine. 2012; 367(9):795-804. [DOI:10.1056/NEJMoa1202753] [PMID]
- Egerházi A, Berecz R, Bartók E, Degrell I. Automated Neuropsychological Test Battery (CANTAB) in mild cognitive impairment and in Alzheimer›s disease. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2007; 31(3):746-51. [DOI:10.1016/j.pnpbp.2007.01.011] [PMID]
- Donix M, Ercoli LM, Siddarth P, Brown JA, Martin-Harris L, Burggren AC, et al. Influence of Alzheimer disease family history and genetic risk on cognitive performance in healthy middle-aged and older people. The American Journal of Geriatric Psychiatry. 2012; 20(7):565-73. [DOI:10.1097/JGP.0b013e3182107e6a] [PMID]
- Caselli RJ, Reiman EM, Osborne D, Hentz JG, Baxter LC, Hernandez JL, et al. Longitudinal changes in cognition and behavior in asymptomatic carriers of the APOE e4 allele. Neurology. 2004; 62(11):1990-5. [DOI:10.1212/01.WNL.0000129533.26544.BF] [PMID]
- Kessels RP, Meulenbroek O, Fernández G, Olde Rikkert MG. Spatial working memory in aging and mild cognitive impairment: effects of task load and contextual cueing. Aging, Neuropsychology, and Cognition. 2010; 17(5):556-74. [DOI:10.1080/13825585.2010.481354] [PMID]
- Kessels RPC, Overbeek A, Bouman Z. Assessment of verbal and visuospatial working memory in mild cognitive impairment and Alzheimer’s dementia. Dementia & Neuropsychologia. 2015; 9(3):301-5. [DOI:10.1590/1980-57642015dn93000014] [PMID]
- Harel BT, Darby D, Pietrzak RH, Ellis KA, Snyder PJ, Maruff P. Examining the nature of impairment in visual paired associate learning in amnestic mild cognitive impairment. Neuropsychology. 2011; 25(6):752-62. [DOI:10.1037/a0024237] [PMID]
- Nanda S, Mohanan N, Kumari S, Mathew M, Ramachandran S, Rajesh Pillai PG, et al. Novel face-name paired associate learning and famous face recognition in mild cognitive impairment: A neuropsychological and brain volumetric study. Dementia and Geriatric Cognitive Disorders Extra.. 2019; 9(1):114-28. [DOI:10.1159/000496476] [PMID]
Type of Study: Original Research |
Subject:
Psychiatry and Psychology
Received: 2024/01/10 | Accepted: 2024/07/31 | Published: 2024/07/31
Received: 2024/01/10 | Accepted: 2024/07/31 | Published: 2024/07/31
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






