Sun, Dec 28, 2025
| فارسی
Volume 29, Issue 3 (Autumn 2023)
IJPCP 2023, 29(3): 320-331 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Saberizafarghandi M B, Vahed N, Arezoomandan R, Pirmoradi M. The Relationship Between Brain Activity and Craving Among Individuals With Cannabis Use Disorder. IJPCP 2023; 29 (3) :320-331
URL: http://ijpcp.iums.ac.ir/article-1-3963-en.html
URL: http://ijpcp.iums.ac.ir/article-1-3963-en.html
1- Department of Addiction, School of Behavioral Sciences and Mental Health (Tehran Institute of Psychiatry), Iran University of Medical Sciences, Tehran, Iran.
2- Research Center for Addiction and Risky Behaviors (ReCARB), Psychosocial Health Research Institute, Iran University of Medical Sciences, Tehran, Iran. ,vahedneda@iums.ac.ir
3- Department of Clinical Psychology, School of Behavioral Sciences and Mental Health (Tehran Institute of Psychiatry), Iran University of Medical Sciences, Tehran, Iran.
2- Research Center for Addiction and Risky Behaviors (ReCARB), Psychosocial Health Research Institute, Iran University of Medical Sciences, Tehran, Iran. ,
3- Department of Clinical Psychology, School of Behavioral Sciences and Mental Health (Tehran Institute of Psychiatry), Iran University of Medical Sciences, Tehran, Iran.
Full-Text [PDF 4204 kb]
(764 Downloads)
| Abstract (HTML) (2209 Views)
Full-Text: (784 Views)
Introduction
Cannabis is the most commonly used illicit substance worldwide, with up to 200 million users. In recent years, cannabis use has increased in Iran, making it the second most commonly used substance after opioids. Prolonged cannabis use is associated with various social, psychiatric, and cognitive consequences. The cannabis use, by stimulating cannabinoid receptors, particularly CB1 receptors, can cause dopaminergic nerve stimulation and dopamine release. Studies have indicated that cannabis use can have long-term negative impact on the brain, causing differences in brain activity between cannabis users and non-users. Addiction to cannabis is characterized by compulsive drug-seeking behavior and craving. The neurobiological mechanism of cannabis craving is still under investigation. Understanding the brain processes involved in cannabis craving can contribute to the development of effective intervention strategies and improve treatment outcomes. There is a need to focus on exploring the neural correlates of cannabis craving using quantitative electroencephalography (QEEG). This study aims to use QEEG to examine the relationship between brain activity and cannabis craving.
Methods
This is a descriptive-analytical study. The study population comprised individuals with cannabis use disorder based on the diagnostic criteria outlined in the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5), referred to the Iran Psychiatric Hospital, of whom 20 were selected based on the entry and exit criteria, using a convenience sampling method. The inclusion criteria were: having cannabis use disorder based on DSM-5 criteria, and age 18-40 years. Exclusion criteria were a history of active brain injury, epilepsy, seizures, trauma, underlying medical conditions, or severe psychiatric disorders (such as schizophrenia, bipolar disorder, and major depressive disorders), concurrent use of psychiatric medications, and concurrent use of substances other than cigarettes. The EEG recordings were obtained from all participants, and the marijuana craving questionnaire-short form was used to assess cravings. The EEG data were recorded using 19 silver cup electrodes referenced to attached ears according to the 10-20 international system, at a rate of 250 Hz. Each recording lasted about 15-20 minutes and was done at a resting state once with closed eyes and once with eyes open. Quantitative analysis of EEG raw data was done using the EEGLAB module of MATLAB software, version 22. The collected data were analyzed using the fast Fourier transform algorithm, and the relative power values were calculated. The assumption of normality for the data distribution was confirmed using the Kolmogorov-Smirnov test, and Pearson correlation test was used to examine the relationship between craving and brain activity.
Results
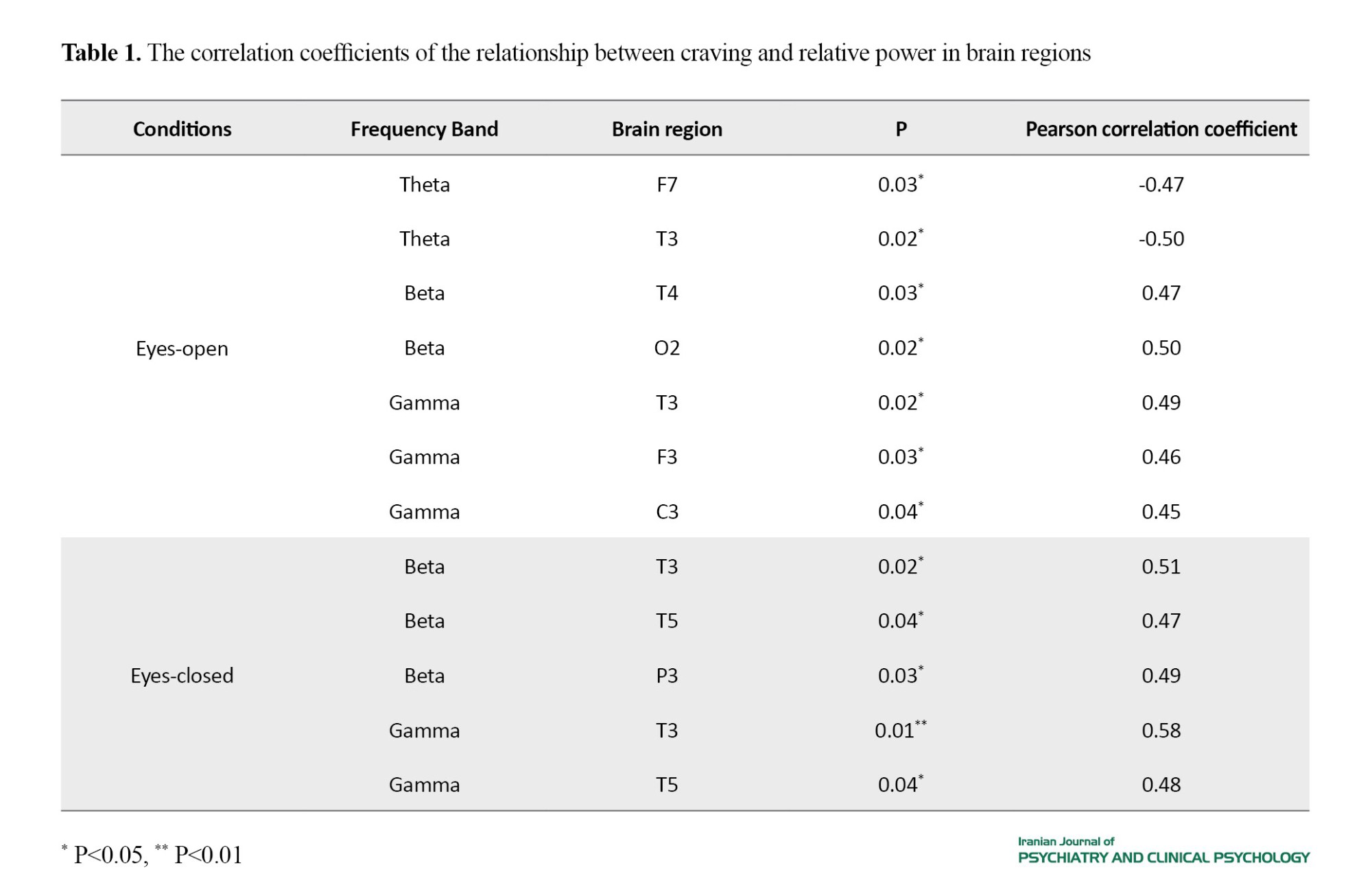
Participants included 19 men and one woman. Table 1 presents the results of correlation test between craving and activity of brain regions at different frequency bands under eyes-open and eyes-closed conditions. A significant negative correlation was observed between craving and relative power at theta frequency band in the frontal (F7) and temporal (T3) regions under eyes-open condition, while there was a significant positive correlation between craving and relative power in the beta frequency band in the temporal (T4) and occipital (O2) regions and in the gamma frequency band in the temporal (T3), frontal (F3), and central (C3) regions. Under eyes-closed condition, a significant positive correlation was found between craving and relative power in the beta and gamma frequency bands in the temporal regions (T3, T5) and parietal region (P3). The results of the linear regression analysis demonstrated that the activity of brain regions was able to predict cravings.
Conclusion
Our findings revealed significant associations between cannabis craving and brain activity in several frequency bands. Consistent with previous studies, we observed that individuals with higher cannabis craving level had lower relative power in the theta band within the frontal and temporal regions. This finding suggests that alterations in power of theta band may be related to the experience of cannabis craving. Moreover, our results indicated that individuals with craving had higher relative power in the beta band within the temporal, occipital, and parietal regions. This increase in beta band power may indicate the impact of cannabis use on working memory and cognitive performance, as well as potential differences in the activity of temporal region. These findings are consistent with existing evidence. For example, fMRI studies reported increased brain activity among cannabis users and a correlation between cannabis craving and the activity of specific brain regions such as the amygdala, striatum, and orbitofrontal cortex.
Furthermore, our study revealed a positive and significant correlation between cannabis craving and relative power in the gamma band within the temporal, frontal, and central regions. This suggests that higher cannabis craving is associated with elevated gamma band power. Interestingly, we did not find any significant correlation between cannabis craving and relative power in the alpha and delta bands. It is important to note that discrepancies in findings may be due to individual differences in factors such as age of onset, dosage and duration of cannabis use, genetics, methodologies, data recording and analysis techniques, EEG devices.
This study provides evidence of a significant relationship between brain activity and craving among individuals with cannabis use disorder. However, further research is needed to enhance our understanding of this relationship and to address the limitations of this study. Continued investigation in this area field can lead to the development of more effective treatment strategies.
Ethical Considerations
Compliance with ethical guidelines
This study has ethical approval from Iran University of Medical Sciences (Code: IR.IUMS.REC.1401.622).
Funding
This article was extracted from the PhD thesis of Neda Vahad, approved by the School of Behavioral Sciences and Mental Health, Tehran Psychiatric Institute. The study was funded by Iran University of Medical Sciences (Grant number: 24915).
Authors contributions
Conceptualization, methodology, data interpretation, writing original draft: Neda Vahad; Supervision, design, review & editing, and funding acquisition: Mohammad Baqer Saberi Zafarkandi and Reza Arzumendan; Counseling: Mohammadreza Pirmoradi; Reading and final approval: All authors.
Conflicts of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank all participants, the Vice-Chancellor for Research of Iran University of Medical Sciences, Iran Psychiatric Hospital, and Dr. Hamidreza Ahmadkhaniha and Dr. Ahmad Hajebi from the Addiction and Risky Behaviors Research Center for cooperation and support in this study.
Cannabis is the most commonly used illicit substance worldwide, with up to 200 million users. In recent years, cannabis use has increased in Iran, making it the second most commonly used substance after opioids. Prolonged cannabis use is associated with various social, psychiatric, and cognitive consequences. The cannabis use, by stimulating cannabinoid receptors, particularly CB1 receptors, can cause dopaminergic nerve stimulation and dopamine release. Studies have indicated that cannabis use can have long-term negative impact on the brain, causing differences in brain activity between cannabis users and non-users. Addiction to cannabis is characterized by compulsive drug-seeking behavior and craving. The neurobiological mechanism of cannabis craving is still under investigation. Understanding the brain processes involved in cannabis craving can contribute to the development of effective intervention strategies and improve treatment outcomes. There is a need to focus on exploring the neural correlates of cannabis craving using quantitative electroencephalography (QEEG). This study aims to use QEEG to examine the relationship between brain activity and cannabis craving.
Methods
This is a descriptive-analytical study. The study population comprised individuals with cannabis use disorder based on the diagnostic criteria outlined in the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5), referred to the Iran Psychiatric Hospital, of whom 20 were selected based on the entry and exit criteria, using a convenience sampling method. The inclusion criteria were: having cannabis use disorder based on DSM-5 criteria, and age 18-40 years. Exclusion criteria were a history of active brain injury, epilepsy, seizures, trauma, underlying medical conditions, or severe psychiatric disorders (such as schizophrenia, bipolar disorder, and major depressive disorders), concurrent use of psychiatric medications, and concurrent use of substances other than cigarettes. The EEG recordings were obtained from all participants, and the marijuana craving questionnaire-short form was used to assess cravings. The EEG data were recorded using 19 silver cup electrodes referenced to attached ears according to the 10-20 international system, at a rate of 250 Hz. Each recording lasted about 15-20 minutes and was done at a resting state once with closed eyes and once with eyes open. Quantitative analysis of EEG raw data was done using the EEGLAB module of MATLAB software, version 22. The collected data were analyzed using the fast Fourier transform algorithm, and the relative power values were calculated. The assumption of normality for the data distribution was confirmed using the Kolmogorov-Smirnov test, and Pearson correlation test was used to examine the relationship between craving and brain activity.
Results
Participants included 19 men and one woman. Table 1 presents the results of correlation test between craving and activity of brain regions at different frequency bands under eyes-open and eyes-closed conditions. A significant negative correlation was observed between craving and relative power at theta frequency band in the frontal (F7) and temporal (T3) regions under eyes-open condition, while there was a significant positive correlation between craving and relative power in the beta frequency band in the temporal (T4) and occipital (O2) regions and in the gamma frequency band in the temporal (T3), frontal (F3), and central (C3) regions. Under eyes-closed condition, a significant positive correlation was found between craving and relative power in the beta and gamma frequency bands in the temporal regions (T3, T5) and parietal region (P3). The results of the linear regression analysis demonstrated that the activity of brain regions was able to predict cravings.

Conclusion
Our findings revealed significant associations between cannabis craving and brain activity in several frequency bands. Consistent with previous studies, we observed that individuals with higher cannabis craving level had lower relative power in the theta band within the frontal and temporal regions. This finding suggests that alterations in power of theta band may be related to the experience of cannabis craving. Moreover, our results indicated that individuals with craving had higher relative power in the beta band within the temporal, occipital, and parietal regions. This increase in beta band power may indicate the impact of cannabis use on working memory and cognitive performance, as well as potential differences in the activity of temporal region. These findings are consistent with existing evidence. For example, fMRI studies reported increased brain activity among cannabis users and a correlation between cannabis craving and the activity of specific brain regions such as the amygdala, striatum, and orbitofrontal cortex.
Furthermore, our study revealed a positive and significant correlation between cannabis craving and relative power in the gamma band within the temporal, frontal, and central regions. This suggests that higher cannabis craving is associated with elevated gamma band power. Interestingly, we did not find any significant correlation between cannabis craving and relative power in the alpha and delta bands. It is important to note that discrepancies in findings may be due to individual differences in factors such as age of onset, dosage and duration of cannabis use, genetics, methodologies, data recording and analysis techniques, EEG devices.
This study provides evidence of a significant relationship between brain activity and craving among individuals with cannabis use disorder. However, further research is needed to enhance our understanding of this relationship and to address the limitations of this study. Continued investigation in this area field can lead to the development of more effective treatment strategies.
Ethical Considerations
Compliance with ethical guidelines
This study has ethical approval from Iran University of Medical Sciences (Code: IR.IUMS.REC.1401.622).
Funding
This article was extracted from the PhD thesis of Neda Vahad, approved by the School of Behavioral Sciences and Mental Health, Tehran Psychiatric Institute. The study was funded by Iran University of Medical Sciences (Grant number: 24915).
Authors contributions
Conceptualization, methodology, data interpretation, writing original draft: Neda Vahad; Supervision, design, review & editing, and funding acquisition: Mohammad Baqer Saberi Zafarkandi and Reza Arzumendan; Counseling: Mohammadreza Pirmoradi; Reading and final approval: All authors.
Conflicts of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank all participants, the Vice-Chancellor for Research of Iran University of Medical Sciences, Iran Psychiatric Hospital, and Dr. Hamidreza Ahmadkhaniha and Dr. Ahmad Hajebi from the Addiction and Risky Behaviors Research Center for cooperation and support in this study.
References
- United Nations. World drug report 2022. New York: United Nations; 2022. [Link]
- Rostam-Abadi Y, Gholami J, Amin-Esmaeili M, Baheshmat S, Hamzehzadeh M, Rafiemanesh H, Nasserbakht M, Ghalichi L, Safarcherati A, Taremian F, Mojtabai R. Evidence for an increase in cannabis use in Iran–A systematic review and trend analysis. PloS One. 2021; 16(8):e0256563. [PMID]
- Foster KT, Arterberry BJ, Iacono WG, McGue M, Hicks BM. Psychosocial functioning among regular cannabis users with and without cannabis use disorder. Psychological Medicine. 2018; 48(11):1853-61. [DOI:10.1017/S0033291717003361] [PMID]
- Weinberger AH, Delnevo CD, Wyka K, Gbedemah M, Lee J, Copeland J, et al. Cannabis use is associated with increased risk of cigarette smoking initiation, persistence, and relapse among adults in the United States. Nicotine and Tobacco Research. 2020; 22(8):1404-8. [PMID]
- Weinberger AH, Wyka K, Goodwin RD. Impact of cannabis legalization in the United States on trends in cannabis use and daily cannabis use among individuals who smoke cigarettes. Drug and Alcohol Dependence. 2022; 238:109563. [DOI:10.1016/j.drugalcdep.2022.109563] [PMID]
- Klumpers LE, Cole DM, Khalili-Mahani N, Soeter RP, Te Beek ET, Rombouts SA, et al. Manipulating brain connectivity with δ9-tetrahydrocannabinol: A pharmacological resting state FMRI study. Neuroimage. 2012; 63(3):1701-11. [DOI:10.1016/j.neuroimage.2012.07.051] [PMID]
- Kopustinskiene DM, Masteikova R, Lazauskas R, Bernatoniene J. Cannabis sativa L. Bioactive compounds and their protective role in oxidative stress and inflammation. Antioxidants. 2022; 11(4):660. [DOI:10.3390/antiox11040660] [PMID]
- Morrison PD, Nottage J, Stone JM, Bhattacharyya S, Tunstall N, Brenneisen R, et al. Disruption of frontal theta coherence by Δ9-tetrahydrocannabinol is associated with positive psychotic symptoms. Neuropsychopharmacology. 2011; 36(4):827-36. [DOI:10.1038/npp.2010.222] [PMID]
- Filbey FM, Aslan S, Calhoun VD, Spence JS, Damaraju E, Caprihan A, et al. Long-term effects of marijuana use on the brain. Proceedings of the National Academy of Sciences of the United States of America. 2014; 111(47):16913-8. [DOI:10.1073/pnas.1415297111] [PMID]
- Prashad S, Dedrick ES, Filbey FM. Cannabis users exhibit increased cortical activation during resting state compared to non-users. NeuroImage. 2018; 179:176-86. [DOI:10.1016/j.neuroimage.2018.06.031] [PMID]
- D’souza DC, Fridberg DJ, Skosnik PD, Williams A, Roach B, Singh N, et al. Dose-related modulation of event-related potentials to novel and target stimuli by intravenous Δ9-THC in humans. Neuropsychopharmacology. 2012; 37(7):1632-46. [DOI:10.1038/npp.2012.8] [PMID]
- Budney AJ, Vandrey RG, Hughes JR, Thostenson JD, Bursac Z. Comparison of cannabis and tobacco withdrawal: Severity and contribution to relapse. Journal of Substance Abuse Treatment. 2008; 35(4):362-8. [DOI:10.1016/j.jsat.2008.01.002] [PMID]
- Fayaz Feyzi Y, Vahed N, Sadeghamal Nikraftar N, Arezoomandan R. Synergistic effect of combined transcranial direct current stimulation and Matrix Model on the reduction of methamphetamine craving and improvement of cognitive functioning: A randomized sham-controlled study. The American Journal of Drug and Alcohol Abuse. 2022; 48(3):311-20. [DOI:10.1080/00952990.2021.2015771] [PMID]
- Budney AJ, Novy PL, Hughes JR. Marijuana withdrawal among adults seeking treatment for marijuana dependence. Addiction. 1999; 94(9):1311-22. [DOI:10.1046/j.1360-0443.1999.94913114.x] [PMID]
- Altıntaş M, İnanç L, Hunca AN, Ektiricioğlu C, Yılmaz N, Tuna ZO, et al. Theory of mind, aggression and impulsivity in patients with synthetic cannabinoid use disorders: A case-control study. Anatolian Journal of Psychiatry. 2019; 20(1):5-12. [Link]
- Chye Y, Kirkham R, Lorenzetti V, McTavish E, Solowij N, Yücel M. Cannabis, cannabinoids, and brain morphology: A review of the evidence. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. 2021; 6(6):627-35. [PMID]
- Chen Y. Online supplemental materials methods (I) QEEG analysis. Journal of Neurology, Neurosurgery, and Psychiatry. 2023; 94:24-9. [Link]
- Heishman SJ, Evans RJ, Singleton EG, Levin KH, Copersino ML, Gorelick DA. Reliability and validity of a short form of the Marijuana Craving Questionnaire. Drug and Alcohol Dependence. 2009; 102(1-3):35-40. [DOI:10.1016/j.drugalcdep.2008.12.010] [PMID]
- Richard CD, Poole JR, McConnell M, Meghdadi AH, Stevanovic-Karic M, Rupp G, et al. Alterations in electroencephalography theta as candidate biomarkers of acute cannabis intoxication. Frontiers in Neuroscience. 2021; 15:744762. [DOI:10.3389/fnins.2021.744762] [PMID]
- Ilan AB, Smith ME, Gevins A. Effects of marijuana on neurophysiological signals of working and episodic memory. Psychopharmacology. 2004; 176(2):214-22. [DOI:10.1007/s00213-004-1868-9] [PMID]
- Herning RI, Better W, Cadet JL. EEG of chronic marijuana users during abstinence: Relationship to years of marijuana use, cerebral blood flow and thyroid function. Clinical Neurophysiology. 2008; 119(2):321-31. [DOI:10.1016/j.clinph.2007.09.140] [PMID]
- Kuhns L, Kroon E, Colyer-Patel K, Cousijn J. Associations between cannabis use, cannabis use disorder, and mood disorders: longitudinal, genetic, and neurocognitive evidence. Psychopharmacology. 2022; 239(5):1231-49. [DOI:10.1007/s00213-021-06001-8] [PMID]
- Sehl H, Terrett G, Greenwood LM, Kowalczyk M, Thomson H, Poudel G, et al. Patterns of brain function associated with cannabis cue-reactivity in regular cannabis users: A systematic review of fMRI studies. Psychopharmacology. 2021; 238(10):2709-28.[DOI:10.1007/s00213-021-05973-x] [PMID]
- Blest-Hopley G, Giampietro V, Bhattacharyya S. Residual effects of cannabis use in adolescent and adult brains-A meta-analysis of fMRI studies. Neuroscience & Biobehavioral Reviews. 2018; 88:26-41. [DOI:10.1016/j.neubiorev.2018.03.008] [PMID]
- Nottage JF, Stone J, Murray RM, Sumich A, Bramon-Bosch E, Ffytche D, et al. Delta-9-tetrahydrocannabinol, neural oscillations above 20 Hz and induced acute psychosis. Psychopharmacology. 2015; 232(3):519-28. [DOI:10.1007/s00213-014-3684-1] [PMID]
- Andriot T, Ohnmacht P, Vuilleumier P, Thorens G, Khazaal Y, Ginovart N, et al. Electrophysiological and behavioral correlates of cannabis use disorder. Cognitive, Affective, & Behavioral Neuroscience. 2022; 22(6):1421-31. [DOI:10.3758/s13415-022-01016-w] [PMID]
- Koukkou M, Lehmann D. Human EEG spectra before and during cannabis hallucinations. Biological Psychiatry. 1976; 11(6):663-77. [PMID]
Type of Study: Original Research |
Subject:
Psychiatry and Psychology
Received: 2023/07/28 | Accepted: 2023/08/18 | Published: 2023/10/1
Received: 2023/07/28 | Accepted: 2023/08/18 | Published: 2023/10/1
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






