Sat, Dec 13, 2025
| فارسی
Volume 29, Issue 3 (Autumn 2023)
IJPCP 2023, 29(3): 250-267 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Dehghani A, Taheri Torbati H R, Sohrabi M, Daneshfar A, Sotoodeh M S. Effects of Online Transcranial Alternating Current Stimulation Over the Parietal Cortex and Supplementary Motor Area on Bimanual Coordination in Elderly Women. IJPCP 2023; 29 (3) :250-267
URL: http://ijpcp.iums.ac.ir/article-1-3892-en.html
URL: http://ijpcp.iums.ac.ir/article-1-3892-en.html
Asiyeh Dehghani1 

 , Hamid Reza Taheri Torbati2
, Hamid Reza Taheri Torbati2 

 , Mehdi Sohrabi1
, Mehdi Sohrabi1 

 , Afkham Daneshfar3
, Afkham Daneshfar3 

 , Mohammad Saber Sotoodeh4
, Mohammad Saber Sotoodeh4 




 , Hamid Reza Taheri Torbati2
, Hamid Reza Taheri Torbati2 

 , Mehdi Sohrabi1
, Mehdi Sohrabi1 

 , Afkham Daneshfar3
, Afkham Daneshfar3 

 , Mohammad Saber Sotoodeh4
, Mohammad Saber Sotoodeh4 


1- Department of Motor Behavior, Faculty of Sports Science, Ferdowsi University of Mashhad, Iran.
2- Department of Motor Behavior, Faculty of Sports Science, Ferdowsi University of Mashhad, Iran. ,hamidtaheri@um.ac.ir
3- Department of Motor Behavior, Faculty of Sport Sciences, Alzahra University, Tehran, Iran.
4- School of Psychology, University of Sussex, Brighton, United Kingdom.
2- Department of Motor Behavior, Faculty of Sports Science, Ferdowsi University of Mashhad, Iran. ,
3- Department of Motor Behavior, Faculty of Sport Sciences, Alzahra University, Tehran, Iran.
4- School of Psychology, University of Sussex, Brighton, United Kingdom.
Full-Text [PDF 6302 kb]
(752 Downloads)
| Abstract (HTML) (2231 Views)
Full-Text: (794 Views)
Introduction
Bimanual coordination in older adults is crucial for participating in daily activities and live independently [1]. Elderly people may experience difficulties in performing activities that require significant bimanual coordination [2]. According to previous studies, the supplementary motor area (SMA) plays an important role in planning and executing sequential and continuous bimanual motor tasks. It also has a crucial role in motor planning prior to movement initiation [3]. The parietal cortex also plays a significant role in bimanual coordination, particularly in complex tasks [4]. Given the changes that occur in old age, providing safe and low-risk solutions to cope with these changes is highly important to reduce the treatment costs and time for elderly people. It has been reported that transcranial alternating current stimulation (tACS), as a non-invasive brain stimulation, facilitates changes in neural activity and promotes neuroplasticity in healthy people [5]. Considering the decline in motor function among the elderly in fine motor tasks and their difficulties performing these tasks in their lives, which are important indicators of functional independence [6], an effective stimulation method to improve bimanual motor skills can be beneficial for the rehabilitation of older adults. Therefore, this study aims to investigate the effects of tACS applied over the right posterior parietal cortex (P4) and SMA on bimanual coordination in elderly women.
Methods
In this quasi-experimental study, 31 healthy elderly women (Mean age 65.26±4.01 years) without a history of neurological or psychological disorders participated. They had normal or corrected-to-normal vision, with no previous experience of tACS. They completed the Montreal Cognitive Assessment Test (MoCA) [7] and the Edinburgh Handedness Inventory (EHI) [8]. The Purdue Pegboard Test (PPT) was used to assess bimanual coordination ability. This task required the participants to use both hands while assembling one pin, one collar, and two washers. They were given a total of 5 minutes to complete the test, and the total score was determined by the number of correctly assembled units at the end of the trial. In this study, a 2-channel brain electrical stimulator (NeuroStim2, Medina Teb, Iran) was used for sham and online brain stimulations. The right parietal cortex (P4) and the SMA (3.0 cm anterior to the central sagittal midline) were identified according to the 10–20 international standard system [9]. The participants received intervention under three conditions: tACS over SMA, tACS over P4, and sham. Each condition was performed in one session with at least a one-week interval. The acquisition phase included four trials of the PPT which lasted 20 min, with a 2-minute interval. During this training, participants received either 20-Hz beta-band tACS or sham stimulation. In the online stimulation protocol, a low intensity of 1 mA at 20 Hz (peak-to-peak intensity) was used. The used waveform was sinusoidal, and each trial lasted for 5 minutes. Throughout the stimulation, each participant underwent four trials, with a 2-minute interval between each trial. For the sham stimulation protocol, the current was gradually increased over a 30-second period at a frequency of 20 Hz, maintained at 1 mA for 10 seconds, and gradually decreased for 30 seconds at 20 Hz. The statistical analyses were conducted in SPSS software, version 26. The normality assumption was assessed using the Shapiro-Wilk test. The sphericity assumption was examined using Mauchly’s test. A repeated measures ANOVA was conducted to determine the difference in the scores of bimanual coordination, followed by a Post hoc test using the Bonferroni test. The significance level was set at 0.05.
Results
Figure 1 shows participants’ bimanual coordination scores in trials 1-4 under three different conditions. According to the results of the Shapiro-Wilk test, the distribution of data was normal (P>0.05), Mauchly’s Test confirmed the sphericity assumption for the main effect of condition (X2(2)=2.878, P=0.237), but not for the main effect of trial (X2(5)=22.558, P<0.001) or the interaction effect of condition and trial (X2(20)=54.197, P<0.001); Therefore, the Greenhouse–Geisser correction was used for the main effect of trial and the interaction effect.
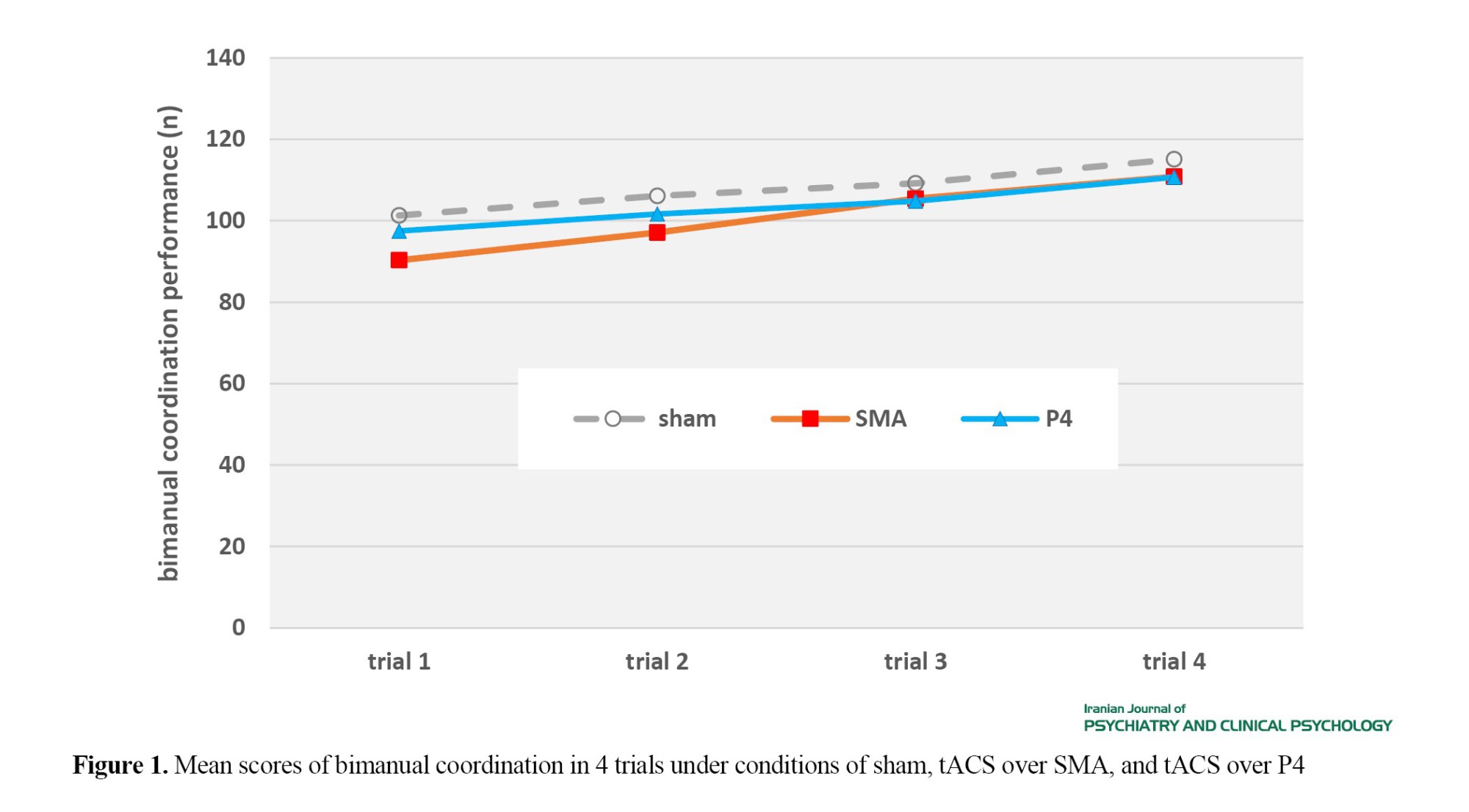
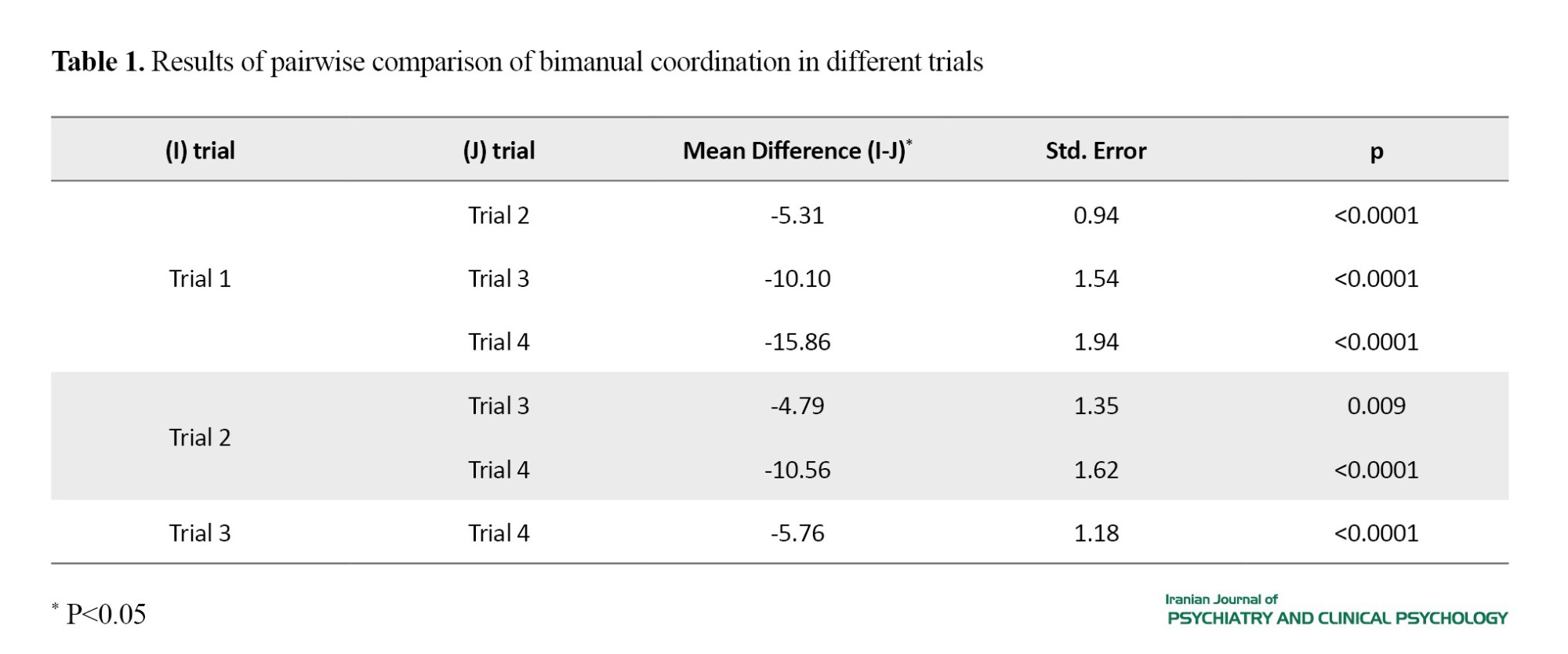
According to the results of ANOVA, the main effect of condition (F(2, 52)=1.867, P=0.165, ŋ2p=0.067) and the interaction effect of condition and trial were not significant (F(3.278, 85.24)=1.855, P=0.138, ŋ2p=0.067), but the main effect of trial was significant (F(1.868,48.562)=42.781, P<0.001, ŋ2p=0.622). Therefore, there was no significant difference in bimanual coordination among different conditions. Based on the results of pairwise comparison using Bonferroni post hoc test for the effect of trial (Table 1), bimanual coordination significantly increased trial-to-trial.
Conclusion
The findings of the present study indicated that active stimulation of the P4 and SMA using β-tACS (20 Hz) did not cause a significant difference in bimanual coordination compared to the sham stimulation. However, the results of pairwise comparisons using Bonferroni post hoc test showed that bimanual coordination significantly improved during the online stimulation. This suggests that although the brain stimulation itself may not have had a significant effect, the training accompanied with it lead to improvements in bimanual coordination. These findings are consistent with previous studies conducted by Berger et al. [10] and Choe et al. [11], but inconsistent with the results of Pogosian et al. [12] and Miyaguchi et al. [13]. A review and meta-analysis study by Hu et al. [14] reported that factors such as stimulation mode, location, and frequency can affect the outcome of tACS regarding motor performance. They also found that online tACS had a significant positive effect on motor performance in healthy individuals compared to sham stimulation. Previous research has shown that tACS can modulate neural activity not only after stimulation but also during stimulation, specifically under the electrode [15]. Furthermore, studies have demonstrated that β-tACS over the motor cortex during task performance can also help stabilize practice related performance improvement. It is worth to note that age may be a factor contributing to the discrepancies between the present study and previous studies. Older adults tend to have decreased dexterity and neural plasticity, which might have eliminated the effects of brain stimulation in this study. Additionally, it is important to consider the duration of brain stimulation. The after-effects of tACS are usually observed over a duration longer than 10 minutes. Given that the present study used only 5 minutes of tACS, it is possible that the after-effects were not fully produced. However, the potential repeated effects of the 5-minute tACS should not be neglected, as suggested by Miyaguchi et al. who used a 1-minute tACS and the PPT on young people [13]. Therefore, further research is needed to fully understand the relationship between tACS-induced changes in neural networks and changes in motor performance. The non-significance effect of tACS on bimanual coordination can be due to the complexity of the bimanual coordination task and the unclear mechanisms underlying bimanual coordination [10]. Some studies have highlighted the importance of beta-band activity in interhemispheric coordination of movements [16], while others did not find any effect of tACS on brain oscillations in the beta frequency [10]. Furthermore, although both the amplitude of motor evoked potentials and the amplitude of beta oscillations in the motor cortex are related to cortical excitability, they do not seem to be strongly related to each other [17]. On the other hand, various studies have shown the importance of the dominant hemisphere in bimanual coordination [18]. However, it is still speculative whether this factor can explain the physiological side effects of tACS without changes in bimanual coordination performance.
In general, according to the findings of the present study, tACS over SMA and P4 had no significant effect on older women’ bimanual coordination. Based on the improvement of bimanual coordination in elderly women after using the Purdue pegboard task, it seems that practice can be safely used to improve bimanual coordination in elderly people. These practices are cost effective and accessible, making them suitable for use at home, rehabilitation centers, and other care centers for the elderly. By improving the quality of life and maintaining the independence of the elderly, these practices can also help reduce the additional costs associated with elderly care.
Ethical Considerations
Compliance with ethical guidelines
The study was approved by the Ethics Committee of Ferdowsi University of Mashhad (code: IR.UM.REC.1400.177). Prior to the study, the participants were given the necessary explanations about the study objectives and procedure and signed a consent form. Their personal information was kept confidential, and they were free to leave the study at any time.
Funding
This study was extracted from the doctoral dissertation of Asiyeh Dehghani, registered by the Faculty of Sports Sciences, Ferdowsi University of Mashhad. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Authors contributions
Conceptualization, design, data collection, data analysis, and writing: Asiyeh Dehghani; Review & editing, methodology, and supervision: All authors.
Conflicts of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank all participants for their cooperation in this study.
Bimanual coordination in older adults is crucial for participating in daily activities and live independently [1]. Elderly people may experience difficulties in performing activities that require significant bimanual coordination [2]. According to previous studies, the supplementary motor area (SMA) plays an important role in planning and executing sequential and continuous bimanual motor tasks. It also has a crucial role in motor planning prior to movement initiation [3]. The parietal cortex also plays a significant role in bimanual coordination, particularly in complex tasks [4]. Given the changes that occur in old age, providing safe and low-risk solutions to cope with these changes is highly important to reduce the treatment costs and time for elderly people. It has been reported that transcranial alternating current stimulation (tACS), as a non-invasive brain stimulation, facilitates changes in neural activity and promotes neuroplasticity in healthy people [5]. Considering the decline in motor function among the elderly in fine motor tasks and their difficulties performing these tasks in their lives, which are important indicators of functional independence [6], an effective stimulation method to improve bimanual motor skills can be beneficial for the rehabilitation of older adults. Therefore, this study aims to investigate the effects of tACS applied over the right posterior parietal cortex (P4) and SMA on bimanual coordination in elderly women.
Methods
In this quasi-experimental study, 31 healthy elderly women (Mean age 65.26±4.01 years) without a history of neurological or psychological disorders participated. They had normal or corrected-to-normal vision, with no previous experience of tACS. They completed the Montreal Cognitive Assessment Test (MoCA) [7] and the Edinburgh Handedness Inventory (EHI) [8]. The Purdue Pegboard Test (PPT) was used to assess bimanual coordination ability. This task required the participants to use both hands while assembling one pin, one collar, and two washers. They were given a total of 5 minutes to complete the test, and the total score was determined by the number of correctly assembled units at the end of the trial. In this study, a 2-channel brain electrical stimulator (NeuroStim2, Medina Teb, Iran) was used for sham and online brain stimulations. The right parietal cortex (P4) and the SMA (3.0 cm anterior to the central sagittal midline) were identified according to the 10–20 international standard system [9]. The participants received intervention under three conditions: tACS over SMA, tACS over P4, and sham. Each condition was performed in one session with at least a one-week interval. The acquisition phase included four trials of the PPT which lasted 20 min, with a 2-minute interval. During this training, participants received either 20-Hz beta-band tACS or sham stimulation. In the online stimulation protocol, a low intensity of 1 mA at 20 Hz (peak-to-peak intensity) was used. The used waveform was sinusoidal, and each trial lasted for 5 minutes. Throughout the stimulation, each participant underwent four trials, with a 2-minute interval between each trial. For the sham stimulation protocol, the current was gradually increased over a 30-second period at a frequency of 20 Hz, maintained at 1 mA for 10 seconds, and gradually decreased for 30 seconds at 20 Hz. The statistical analyses were conducted in SPSS software, version 26. The normality assumption was assessed using the Shapiro-Wilk test. The sphericity assumption was examined using Mauchly’s test. A repeated measures ANOVA was conducted to determine the difference in the scores of bimanual coordination, followed by a Post hoc test using the Bonferroni test. The significance level was set at 0.05.
Results
Figure 1 shows participants’ bimanual coordination scores in trials 1-4 under three different conditions. According to the results of the Shapiro-Wilk test, the distribution of data was normal (P>0.05), Mauchly’s Test confirmed the sphericity assumption for the main effect of condition (X2(2)=2.878, P=0.237), but not for the main effect of trial (X2(5)=22.558, P<0.001) or the interaction effect of condition and trial (X2(20)=54.197, P<0.001); Therefore, the Greenhouse–Geisser correction was used for the main effect of trial and the interaction effect.

According to the results of ANOVA, the main effect of condition (F(2, 52)=1.867, P=0.165, ŋ2p=0.067) and the interaction effect of condition and trial were not significant (F(3.278, 85.24)=1.855, P=0.138, ŋ2p=0.067), but the main effect of trial was significant (F(1.868,48.562)=42.781, P<0.001, ŋ2p=0.622). Therefore, there was no significant difference in bimanual coordination among different conditions. Based on the results of pairwise comparison using Bonferroni post hoc test for the effect of trial (Table 1), bimanual coordination significantly increased trial-to-trial.

Conclusion
The findings of the present study indicated that active stimulation of the P4 and SMA using β-tACS (20 Hz) did not cause a significant difference in bimanual coordination compared to the sham stimulation. However, the results of pairwise comparisons using Bonferroni post hoc test showed that bimanual coordination significantly improved during the online stimulation. This suggests that although the brain stimulation itself may not have had a significant effect, the training accompanied with it lead to improvements in bimanual coordination. These findings are consistent with previous studies conducted by Berger et al. [10] and Choe et al. [11], but inconsistent with the results of Pogosian et al. [12] and Miyaguchi et al. [13]. A review and meta-analysis study by Hu et al. [14] reported that factors such as stimulation mode, location, and frequency can affect the outcome of tACS regarding motor performance. They also found that online tACS had a significant positive effect on motor performance in healthy individuals compared to sham stimulation. Previous research has shown that tACS can modulate neural activity not only after stimulation but also during stimulation, specifically under the electrode [15]. Furthermore, studies have demonstrated that β-tACS over the motor cortex during task performance can also help stabilize practice related performance improvement. It is worth to note that age may be a factor contributing to the discrepancies between the present study and previous studies. Older adults tend to have decreased dexterity and neural plasticity, which might have eliminated the effects of brain stimulation in this study. Additionally, it is important to consider the duration of brain stimulation. The after-effects of tACS are usually observed over a duration longer than 10 minutes. Given that the present study used only 5 minutes of tACS, it is possible that the after-effects were not fully produced. However, the potential repeated effects of the 5-minute tACS should not be neglected, as suggested by Miyaguchi et al. who used a 1-minute tACS and the PPT on young people [13]. Therefore, further research is needed to fully understand the relationship between tACS-induced changes in neural networks and changes in motor performance. The non-significance effect of tACS on bimanual coordination can be due to the complexity of the bimanual coordination task and the unclear mechanisms underlying bimanual coordination [10]. Some studies have highlighted the importance of beta-band activity in interhemispheric coordination of movements [16], while others did not find any effect of tACS on brain oscillations in the beta frequency [10]. Furthermore, although both the amplitude of motor evoked potentials and the amplitude of beta oscillations in the motor cortex are related to cortical excitability, they do not seem to be strongly related to each other [17]. On the other hand, various studies have shown the importance of the dominant hemisphere in bimanual coordination [18]. However, it is still speculative whether this factor can explain the physiological side effects of tACS without changes in bimanual coordination performance.
In general, according to the findings of the present study, tACS over SMA and P4 had no significant effect on older women’ bimanual coordination. Based on the improvement of bimanual coordination in elderly women after using the Purdue pegboard task, it seems that practice can be safely used to improve bimanual coordination in elderly people. These practices are cost effective and accessible, making them suitable for use at home, rehabilitation centers, and other care centers for the elderly. By improving the quality of life and maintaining the independence of the elderly, these practices can also help reduce the additional costs associated with elderly care.
Ethical Considerations
Compliance with ethical guidelines
The study was approved by the Ethics Committee of Ferdowsi University of Mashhad (code: IR.UM.REC.1400.177). Prior to the study, the participants were given the necessary explanations about the study objectives and procedure and signed a consent form. Their personal information was kept confidential, and they were free to leave the study at any time.
Funding
This study was extracted from the doctoral dissertation of Asiyeh Dehghani, registered by the Faculty of Sports Sciences, Ferdowsi University of Mashhad. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Authors contributions
Conceptualization, design, data collection, data analysis, and writing: Asiyeh Dehghani; Review & editing, methodology, and supervision: All authors.
Conflicts of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank all participants for their cooperation in this study.
References
- Brayne C, Miller B. Dementia and aging populations-A global priority for contextualized research and health policy. PLoS Medicine. 2017; 14(3):e1002275. [DOI:10.1371/journal.pmed.1002275] [PMID]
- Lin CH, Chou LW, Wei SH, Lieu FK, Chiang SL, Sung WH. Influence of aging on bimanual coordination control. Experimental Gerontology. 2014; 53:40-7. [DOI:10.1016/j.exger.2014.02.005] [PMID]
- Abedan Zadeh R, Abdoli B, Farsi AR. [The effect of the sensory information and age on the transition of the relative phase in bimanual coordination pattern (Perian)]. Jundishapur Scientific Medical Journal. 2017; 15(6):619-33. [Link]
- Maes C, Gooijers J, Orban de Xivry JJ, Swinnen SP, Boisgontier MP. Two hands, one brain, and aging. Neuroscience & Biobehavioral Reviews. 2017; 75:234-56. [DOI:10.1016/j.neubiorev.2017.01.052] [PMID]
- Temprado JJ, Torre MM, Langeard A, Julien-Vintrou M, Devillers-Réolon L, Sleimen-Malkoun R, et al. Intentional switching between bimanual coordination patterns in older adults: Is it mediated by inhibition processes? Frontiers in Aging Neuroscience. 2020; 12:29. [DOI:10.3389/fnagi.2020.00029] [PMID]
- Grefkes C, Eickhoff SB, Nowak DA, Dafotakis M, Fink GR. Dynamic intra-and interhemispheric interactions during unilateral and bilateral hand movements assessed with fMRI and DCM. Neuroimage. 2008; 41(4):1382-94. [DOI:10.1016/j.neuroimage.2008.03.048] [PMID]
- Toyokura M, Muro I, Komiya T, Obara M. Activation of pre-supplementary motor area (SMA) and SMA proper during unimanual and bimanual complex sequences: An analysis using functional magnetic resonance imaging. Journal of Neuroimaging. 2002; 12(2):172-8. [DOI:10.1111/j.1552-6569.2002.tb00116.x] [PMID]
- Taube W, Mouthon M, Leukel C, Hoogewoud HM, Annoni JM, Keller M. Brain activity during observation and motor imagery of different balance tasks: An fMRI study. Cortex. 2015; 64:102-14. [DOI:10.1016/j.cortex.2014.09.022] [PMID]
- Stephan KM, Fink GR, Passingham RE, Silbersweig D, Ceballos-Baumann AO, Frith CD, et al. Functional anatomy of the mental representation of upper extremity movements in healthy subjects. Journal of Neurophysiology. 1995; 73(1):373-86. [DOI:10.1152/jn.1995.73.1.373] [PMID]
- Serrien DJ, Strens LH, Oliviero A, Brown P. Repetitive transcranial magnetic stimulation of the supplementary motor area (SMA) degrades bimanual movement control in humans. Neuroscience Letters. 2002; 328(2):89-92. [DOI:10.1016/S0304-3940(02)00499-8] [PMID]
- Ramnani N, Toni I, Passingham RE, Haggard P. The cerebellum and parietal cortex play a specific role in coordination: A PET study. Neuroimage. 2001; 14(4):899-911. [DOI:10.1006/nimg.2001.0885] [PMID]
- Pergolizzi D. The role of posterior parietal cortex in episodic memory retrieval: Transcranial Direct Current Stimulation Studies (tDCS) [PhD dissertation]. New York: City University of New York; 2015. [Link]
- Huk AC, Meister ML. Neural correlates and neural computations in posterior parietal cortex during perceptual decision-making. Frontiers in Integrative Neuroscience. 2012; 6:86. [DOI:10.3389/fnint.2012.00086] [PMID]
- Mooshagian E, Kaplan J, Zaidel E, Iacoboni M. Fast visuomotor processing of redundant targets: The role of the right temporo-parietal junction. PLoS One. 2008; 3(6):e2348. [DOI:10.1371/journal.pone.0002348] [PMID]
- Azarpaikan A, Torbati H, Sohrabi M, Boostani R, Ghoshuni M. The effect of parietal and cerebellar transcranial direct current stimulation on bimanual coordinated adaptive motor learning. Journal of Psychophysiology. 2019. [Link]
- Yeghaneh B, Einalia J, Charaghi M, Eskandari Shahraki Z. [Evaluation of quality of life and vulnerability components of elderly women in rural areas case study: Zanjan city (Persian)]. Journal of Gerontology. 2019; 3(4):67-77. [Link]
- Farokhnezhad Afshar P, Malakouti SK, Rashedi V, Ajri-khameslou M. [Relationship between place attachment and social functioning in the elderly (Persian)]. Iranian Journal of Psychiatry and Clinical Psychology. 2021; 27(2):194-203. [DOI:10.32598/ijpcp.27.2.2822.2]
- Akbari Kamrani AA, Azadi F, Foroughan M, Siadat S, Kaldi AR. [Characteristics of falls among institutionalized elderly people (Persian)]. Salmand: Iranian Journal of Ageing. 2007; 1(2):101-5. [Link]
- Fathi-Rezaie Z, Aslankhani MA, Farsi A, Abdoli B, Zamani-Sani SH. [A comparison of three functional tests of balance in identifying fallers from non-fallers in elderly people (Persian)]. Knowledge & Health. 2010; 4(4):22-7. [Link]
- Suzuki M, Tanaka S, Gomez-Tames J, Okabe T, Cho K, Iso N, et al. Nonequivalent after-effects of alternating current stimulation on motor cortex oscillation and inhibition: Simulation and experimental study. Brain Sciences. 2022; 12(2):195. [DOI:10.3390/brainsci12020195] [PMID]
- Helfrich RF, Schneider TR, Rach S, Trautmann-Lengsfeld SA, Engel AK, Herrmann CS. Entrainment of brain oscillations by transcranial alternating current stimulation. Current Biology. 2014; 24(3):333-9. [DOI:10.1016/j.cub.2013.12.041] [PMID]
- Miyaguchi S, Inukai Y, Takahashi R, Miyashita M, Matsumoto Y, Otsuru N, et al. Effects of stimulating the supplementary motor area with a transcranial alternating current for bimanual movement performance. Behavioural Brain Research. 2020; 393:112801. [DOI:10.1016/j.bbr.2020.112801] [PMID]
- Berger A, Pixa NH, Steinberg F, Doppelmayr M. Brain oscillatory and hemodynamic activity in a bimanual coordination task following transcranial alternating current stimulation (tACS): A combined EEG-fNIRS study. Frontiers in Behavioral Neuroscience. 2018; 12:67. [DOI:10.3389/fnbeh.2018.00067] [PMID]
- Azarpaikan A, Taherii Torbati H, Sohrabi M, Boostani R, Ghoshuni M. Power spectral parameter variations after transcranial direct current stimulation in a bimanual coordination task. Adaptive Behavior. 2021; 29(1):25-38. [DOI:10.1177/1059712319879971]
- Miyaguchi S, Inukai Y, Mitsumoto S, Otsuru N, Onishi H. Gamma-transcranial alternating current stimulation on the cerebellum and supplementary motor area improves bimanual motor skill. Behavioural Brain Research. 2022; 424:113805. [DOI:10.1016/j.bbr.2022.113805] [PMID]
- Sugata H, Yagi K, Yazawa S, Nagase Y, Tsuruta K, Ikeda T, et al. Modulation of motor learning capacity by transcranial alternating current stimulation. Neuroscience. 2018; 391:131-9. [DOI:10.1016/j.neuroscience.2018.09.013] [PMID]
- Miyaguchi S, Otsuru N, Kojima S, Saito K, Inukai Y, Masaki M, et al. Transcranial alternating current stimulation with gamma oscillations over the primary motor cortex and cerebellar hemisphere improved visuomotor performance. Frontiers in Behavioral Neuroscience. 2018; 12:132. [DOI:10.3389/fnbeh.2018.00132] [PMID]
- Hu K, Wan R, Liu Y, Niu M, Guo J, Guo F. Effects of transcranial alternating current stimulation on motor performance and motor learning for healthy individuals: A systematic review and meta-analysis. Frontiers in Physiology. 2022; 13:1064584. [PMID]
- Alipour A, Agah Haris M. [Reliability and validity of Edinburg Handedness Inventory in Iran (Persian)]. Journal of Modern Psychological Researches. 2007; 7(26):110-31. [Link]
- Sikaroodi H, Majidi A, Samadi S, Shirzad H, Aghdam H, Azimi Kia A, et al. [Evaluating reliability of the montreal cognitive assessment test and its agreement with neurologist diagnosed among patients with cognitive complaints (Persian)]. Journal of Police Medicine. 2012; 1(1):11-7. [Link]
- Arastoo AA, Zahednejad S, Parsaei S, Alboghebish S, Ataei N, Ameriasl H. [The effect of direct current stimulation in left dorsolateral prefrontal cortex on working memory in veterans and disabled athletes (Persian)]. Daneshvar Medicine. 2020; 26(6):25-32. [Link]
- Green PE, Ridding MC, Hill KD, Semmler JG, Drummond PD, Vallence AM. Supplementary motor area-primary motor cortex facilitation in younger but not older adults. Neurobiology of Aging. 2018; 64:85-91. [DOI:10.1016/j.neurobiolaging.2017.12.016] [PMID]
- Manji A, Amimoto K, Matsuda T, Wada Y, Inaba A, Ko S. Effects of transcranial direct current stimulation over the supplementary motor area body weight-supported treadmill gait training in hemiparetic patients after stroke. Neuroscience Letters. 2018; 662:302-5. [DOI:10.1016/j.neulet.2017.10.049] [PMID]
- Mehdizadeh H, Taghizadeh G, Ashayeri HA. [Test-retest reliability of the Purdue Pegboard test in drug on-phase for patients with Parkinson's disease (Persian)]. Koomesh. 2010; 11(3):189-97. [Link]
- Rjosk V, Kaminski E, Hoff M, Gundlach C, Villringer A, Sehm B, et al. Transcranial alternating current stimulation at beta frequency: Lack of immediate effects on excitation and interhemispheric inhibition of the human motor cortex. Frontiers in Human Neuroscience. 2016; 10:560. [DOI:10.3389/fnhum.2016.00560] [PMID]
- Serrien DJ, Cassidy MJ, Brown P. The importance of the dominant hemisphere in the organization of bimanual movements. Human Brain Mapping. 2003; 18(4):296-305. [DOI:10.1002/hbm.10086] [PMID]
- Davis NJ, Tomlinson SP, Morgan HM. The role of Beta-frequency neural oscillations in motor control. Journal of Neuroscience. 2012; 32(2):403-4. [DOI:10.1523/JNEUROSCI.5106-11.2012]
- Rumpf JJ, Barbu A, Fricke C, Wegscheider M, Classen J. Posttraining Alpha transcranial alternating current stimulation impairs motor consolidation in elderly people. Neural Plasticity. 2019; 2019:2689790. [DOI:10.1155/2019/2689790] [PMID]
- Choe J, Coffman BA, Bergstedt DT, Ziegler MD, Phillips ME. Transcranial direct current stimulation modulates neuronal activity and learning in pilot training. Frontiers in Human Neuroscience. 2016; 10:34. [DOI:10.3389/fnhum.2016.00034] [PMID]
- Heise KF, Monteiro T, Gijbels V, Leunissen I, Mantni D, Swinnen S. Modulation of interhemispheric connectivity by alternating current stimulation and its impact on transitions between bimanual movements of varying stability. Brain Stimulation: Basic, Translational, and Clinical Research in Neuromodulation. 2017; 10(2):452. [Link]
- Krause V, Meier A, Dinkelbach L, Pollok B. Beta band transcranial alternating (tACS) and direct current stimulation (tDCS) applied after initial learning facilitate retrieval of a motor sequence. Frontiers in Behavioral Neuroscience. 2016; 10:4. [DOI:10.3389/fnbeh.2016.00004] [PMID]
- Pogosyan A, Gaynor LD, Eusebio A, Brown P. Boosting cortical activity at beta-band frequencies slows movement in humans. Current Biology. 2009; 19(19):1637-41. [DOI:10.1016/j.cub.2009.07.074] [PMID]
- Pollok B, Boysen AC, Krause V. The effect of transcranial alternating current stimulation (tACS) at alpha and beta frequency on motor learning. Behavioural Brain Research. 2015; 293:234-40. [DOI:10.1016/j.bbr.2015.07.049] [PMID]
- Helfrich RF, Knepper H, Nolte G, Strüber D, Rach S, Herrmann CS, et al. Selective modulation of interhemispheric functional connectivity by HD-tACS shapes perception. PLoS Biology. 2014; 12(12):e1002031. [DOI:10.1371/journal.pbio.1002031] [PMID]
- Kasten FH, Dowsett J, Herrmann CS. Sustained aftereffect of α-tACS lasts up to 70 min after stimulation. Frontiers in Human Neuroscience. 2016; 10:245. [DOI:10.3389/fnhum.2016.00245] [PMID]
- Veniero D, Vossen A, Gross J, Thut G. Lasting EEG/MEG aftereffects of rhythmic transcranial brain stimulation: Level of control over oscillatory network activity. Frontiers in Cellular Neuroscience. 2015; 9:477. [DOI:10.3389/fncel.2015.00477] [PMID]
- Pixa NH, Berger A, Steinberg F, Doppelmayr M. Parietal, but not motor cortex, HD-atDCS deteriorates learning transfer of a complex bimanual coordination task. Journal of Cognitive Enhancement. 2019; 3:111-23. [DOI:10.1007/s41465-018-0088-x]
- Wischnewski M, Engelhardt M, Salehinejad MA, Schutter DJLG, Kuo MF, Nitsche MA. NMDA receptor-mediated motor cortex plasticity after 20 Hz transcranial alternating current stimulation. Cerebral Cortex. 2019; 29(7):2924-31. [DOI:10.1093/cercor/bhy160] [PMID]
- Mäki H, Ilmoniemi RJ. EEG oscillations and magnetically evoked motor potentials reflect motor system excitability in overlapping neuronal populations. Clinical Neurophysiology. 2010; 121(4):492-501. [DOI:10.1016/j.clinph.2009.11.078] [PMID]
- Kennerley SW, Diedrichsen J, Hazeltine E, Semjen A, Ivry RB. Callosotomy patients exhibit temporal uncoupling during continuous bimanual movements. Nature Neuroscience. 2002; 5(4):376-81. [DOI:10.1038/nn822] [PMID]
- Serrien DJ, Ivry RB, Swinnen SP. Dynamics of hemispheric specialization and integration in the context of motor control. Nature Reviews Neuroscience. 2006; 7(2):160-6. [DOI:10.1038/nrn1849] [PMID]
- Haaland KY, Harrington DL. Hemispheric control of the initial and corrective components of aiming movements. Neuropsychologia. 1989; 27(7):961-9. [DOI:10.1016/0028-3932(89)90071-7] [PMID]
Type of Study: Original Research |
Subject:
Psychiatry and Psychology
Received: 2023/04/30 | Accepted: 2023/09/2 | Published: 2023/10/1
Received: 2023/04/30 | Accepted: 2023/09/2 | Published: 2023/10/1
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |



